Answers
Answer:
\(1) \: 1.644 kg = 1.64kg \: in \: three \: significant \: figures. \\ 2) \: 74.2587 L =74.3L \: in \: three \: significant figures. \\
3) 0.00268756 cm \: = 0.00269cm \: in \: three \: significant \: figures. \\ 4) 8907 m = 8910m \: in \: three \: significant \: figures.\)
Related Questions
Natural gas is a natural resource used by Americans for heating and for generating electricity. If individual Americans continue to each use the same amount of natural gas each year, then which of the following will also be true?
A.
Natural gas will become the most commonly used energy resource in the U.S.
B.
The amount of natural gas consumed in the U.S. will change as the population changes.
C.
The amount of natural gas consumed in the U.S. will remain constant even if the population size changes.
Answers
Answer:
C. The amount of natural gas consumed in the U.S. will remain constant even if the population size changes.
characteristics. of. rusting
Answers
Answer: metal turn orange and weaker as it gets oxidised
Explanation:
Suppose that the microwave radiation has a wavelength of 12.4 cm . How many photons are required to heat 255 mL of coffee from 25.0 ∘C to 62.0 ∘C
Answers
Answer:
Explanation:
wavelength λ = 12.4 x 10⁻² m .
energy of one photon = h c / λ
= 6.6 x 10⁻³⁴ x 3 x 10⁸ / 12.4 x 10⁻²
= 1.6 x 10⁻²⁴ J .
Let density of coffee be equal to density of water .
mass of coffee = 255 x 1 = 255 g
heat required to heat up coffee = mass x specific heat x rise in temp
= 255 x 4.18 x ( 62-25 )
= 39438.3 J .
No of photons required = heat energy required / energy of one photon
= 39438.3 / 1.6 x 10⁻²⁴
= 24649 x 10²⁴
= 24.65 x 10²⁷ .
Consider the reaction 4NH3 + 5O2 4NO + 6H2O. In an experiment, 3.25 g of NH3 are allowed to react with 3.50 g of O2. a) Which reactant is the limiting reagent? _________________ b) How many grams of NO are formed? _________________ c) How many grams of the excess reactant remains after the reaction?
Answers
In order to find the limiting reactant we will need to first set up the properly balance equation, which the question already provided us:
4 NH3 + 5 O2 -> 4 NO + 6 H2O
Now from the information in the question we have:
3.25 g of NH3
3.50 g of O2
We will need their molar mass in order to find how many moles of each compound we actually have:
O2 = 32 g/mol
NH3 = 17.03 g/mol
As we can see, in a regular reaction, we will have a 4:5 molar ratio between NH3 and O2, which means that for every 4 moles of NH3 we will need 5 moles of O2 in order to proceed in the reaction, not let's find the number of moles in NH3
17.03 g = 1 mol
3.25 g = x moles
x = 0.19 moles of NH3
Now we will use the molar ratio to find the number of moles of O2
4 NH3 = 5 O2
0.19 NH3 = x O2
x = 0.24 moles
Now we need to check if 0.24 moles of O2 is under the 3.50 grams of mass, if the mass is below 3.50, we have an excess of O2, therefore the limiting reactant will be NH3, if we have a mass above 3.50, this means that we actually have an excess of NH3, and the limiting reactant is O2
32 g = 1 mol
x grams = 0.24 moles
x = 7.68 grams, which is above 3.50, which means that the limiting reactant is O2 and we have an excess of NH3
A) Oxygen gas, O2
Now for letter B
We have to use the limiting reactant to find out how much is the mass of the product, so let's use O2, which has 3.50 g of mass and molar mass of 32 g/mol
32 g = 1 mol
3.50 g = x moles
x = 0.11 moles of O2
The molar ratio is 5:4, 5 moles of Oxygen gas for 4 moles of NO, this in a regular situation, but we have 0.11 moles of O2, for NO will be:
5 O2 = 4 NO
0.11 O2 = x NO
x = 0.09 moles of NO
Now we have to find the mass, we will use its molar mass, which is 30.01 g/mol
30.01 g = 1 mol
x grams = 0.09 moles
x = 2.7 grams of NO
b) 2.7 grams of NO
For letter C
We already know that the oxygen gas is the limiting reactant, and we have 0.11 moles of O2 in the reaction, let's find out how many grams are in excess in 3.25 g of NH3, again we are going to use molar ratio 4:5
4 NH3 = 5 O2
x NH3 = 0.11 O2
x = 0.09 moles of NH3
Using the molar mass of NH3 we will end up with
17.03 g = 1 mol
x grams = 0.09 moles
x = 1.53 grams of NH3
Now we only need to subtract this from 3.25 g
3.25 - 1.53
We have an excess of 1.72 grams of NH3
c) 1.72 grams of NH3
2. Which number is not a coefficient in the equation,
2C6H14+ 19O2,-- 12CO2,+ 14H2O?
Answers
Answer:
2, 19, 12 and 14 are the coefficients.
The volume of a gas 100mmHg pressure and at 40°C is 480mL. What volume does the gas occupy at standard temperature and pressure ?
Answers
Explanation:
293८4639374493७47392946
Answer:
The volume of the gas at standard temperature and pressure is 378 mL.
Step-by-step Explanation:
To solve this problem, we can use the combined gas law, which relates the pressure, volume, and temperature of a gas:
P1V1/T1 = P2V2/T2
where P1, V1, and T1 are the initial pressure, volume, and temperature, respectively, and P2, V2, and T2 are the final pressure, volume, and temperature, respectively.
We know that the gas has an initial pressure of 100 mmHg and an initial volume of 480 mL at a temperature of 40°C. We want to find the final volume of the gas at standard temperature and pressure, which is defined as 0°C and 1 atm (or 760 mmHg) of pressure.
First, we need to convert the initial temperature from Celsius to Kelvin by adding 273.15:
T1 = 40°C + 273.15 = 313.15 K
Next, we can plug in the values into the combined gas law equation:
(100 mmHg)(480 mL)/(313.15 K) = (760 mmHg)(V2)/(273.15 K)
We can solve for V2 by cross-multiplying and simplifying:
V2 = (100 mmHg)(480 mL)(273.15 K)/(313.15 K)(760 mmHg)
= 378 mL
Therefore, the volume of the gas at standard temperature and pressure is 378 mL.
What is the final temperature after 840 Joules is absorbed by 10.0g of water at 25.0
C?
Answers
The final temperature of the water is: T_final = 45.0°C
We can use the formula for the specific heat capacity of the water to solve this problem:
q = mcΔT
First, we can calculate the initial energy of the water:
q = mcΔT
q = (10.0 g) (4.184 J/g°C) (25.0°C)
q = 1,046 J
Next, we can calculate the final temperature after absorbing 840 J:
q = mcΔT
840 J = (10.0 g) (4.184 J/g°C) (ΔT)
ΔT = 20.0°C
Therefore, the final temperature of the water is:
T_final = T_initial + ΔT
T_final = 25.0°C + 20.0°C
T_final = 45.0°C
To know more about final temperature, here
brainly.com/question/11244611
#SPJ1
ASAP PLEASE!!!B. Complete the drawing for the sample reaction below to show the law of conservation of
mass, when XY is produced.
+
->
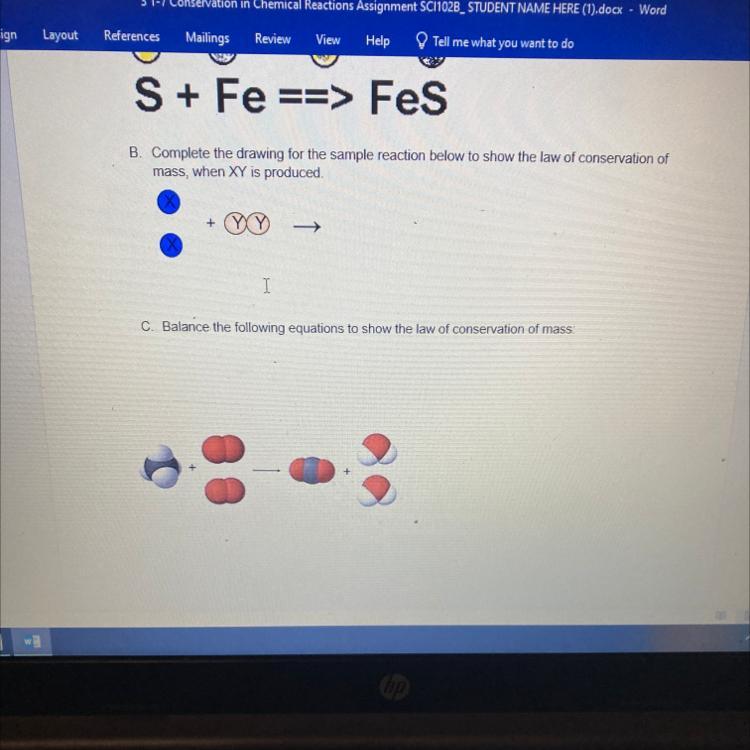
Answers
The complete reaction, according to the law of conservation of mass is:
XX + YY → 2XY
The Law of Conservation is a fundamental principle in chemistry and physics. It states that in a closed system, mass cannot be created or destroyed during a chemical reaction or a physical change. The total mass of the substances involved before the reaction or change must equal the total mass of the substances after the reaction or change.
This principle is based on the understanding that atoms are not created or destroyed, but they can combine or separate to form different substances.
Learn more about the law of mass conservation, here:
https://brainly.com/question/28711001
#SPJ1
Question 5 of 10 Large telescopes have revealed information about the motions of stars. They helped scientists realize that most stars and galaxies are accelerating away from one another. This led scientists to begin to develop the big bang theory. This new theory explained that the universe began as an enormous explosion outward from a single point. The work described in the passage is part of which process used in developing scientific theories? O A. Using imagination to modify existing technology B. Using observations to spark an idea C. Using data to develop new technology D. Using new technology to modify an existing theory
Answers
Answer: using observations to spark an idea
Explanation:
Answer:
B
Explanation:
B
1. Which of the following statements about carbon oxides is FALSE?
Answers
Answer:
what are the following statements?
Explanation:
A molecule contains 47.30 g mercury (Hg) and 16.70 (Cl). what is its empirical formula?

Answers
Explanation:
Convert grams to moles for both elements by dividing the mass by it’s molar mass (Mr).
Hg- 47.30/201= 0.23 mols
Cl- 16.70/35= 0.47 mols
Now, divide the moles of both elements with the smallest number. The smallest number here is 0.23.
Hg- 0.23/0.23= 1
Cl- 0.47/0.23= 2.04 (since there is a 0 after the decimal we will forget the decimal and write the number as a whole.)
Empirical Formula= Hg1Cl2

WILL AWARD BRAINLIEST!!! A chemist performs a reaction combining 100.1 g of aluminum chloride (AlCl3), and 57.2 g of sodium hydroxide (NaOH). The products are aluminum hydroxide (Al(OH)3) and sodium chloride (NaCl).
a. Please write out the balanced equation for this reaction.
b. Assuming all the aluminum chloride is consumed in this reaction, how many grams of aluminum hydroxide will form? Work must be shown to earn credit.
c. If all the sodium hydroxide is used up, how many grams of aluminum hydroxide can be formed? Work must be shown to earn credit.
d. What is the theoretical yield of this reaction? Which reagent is limiting? Explain your answer.
Answers
a.The balanced reaction between \(AlCl_3\) and NaOH is:
\(AlCl_3 + 3NaOH\) → \(Al(OH)_3 + 3NaCl\) is the balanced reaction.
b.58.55g of aluminum hydroxide will form? Work must be shown to earn credit
c. 6.50 g grams of aluminum hydroxide can be formed.
d.\(Al(OH)_3\) produced from NaOH is less, NaOH will be the limiting reagent. The amount of \(Al(OH)_3\) actually produced is 4.13 g.
What is a balanced chemical equation?An equation that has an equal number of atoms of each element on both sides of the equation is called a balanced chemical equation.
a.The balanced reaction between \(AlCl_3\) and NaOH is:
\(AlCl_3 + 3NaOH\) → \(Al(OH)_3 + 3NaCl\) is the balanced reaction.
b) Given mass of \(AlCl_3\) = 100.1 g
Molar mass of \(AlCl_3\) = 133.34 g/mol
moles of \(AlCl_3\) = 100.1 g ÷133.34 = 0.7507 moles
Based on the reaction stoichiometry:
1 mole of \(AlCl_3\) produces 1 mole of \(Al(OH)_3\)
# moles of \(Al(OH)_3\) = 0.7507 moles
Molar mass \(Al(OH)_3\) = 78 g/mol
Amount of \(Al(OH)_3\) produced = 0.7507 moles x 78 = 58.55g
c) Given mass of NaOH = 57.2 g
Molar mass of NaOH= 40 g/mol
moles of NaOH = 57.2 g ÷ 40 = 1.43 moles
Based on the reaction stoichiometry:
3 moles of NaOH produces 1 mole of \(Al(OH)_3\)
moles of \(Al(OH)_3\) produced = 1.43÷ 3 = 0.4766 moles
Molar mass \(Al(OH)_3\) = 78 g/mol
Amount of \(Al(OH)_3\) produced = 0.4766 78 = 6.50 g
d) Since moles of \(Al(OH)_3\) produced from NaOH is less, NaOH will be the limiting reagent. The amount of \(Al(OH)_3\) actually produced is 6.50 g.
Learn more about balanced chemical equations here:
https://brainly.com/question/12053181
#SPJ1
How do you know the correct number of significant figures to include in an answer?
Answers
Answer:
Explanation:
1 Non-zero digits are always significant.
2 Any zeros between two significant digits are significant.
3 A final zero or trailing zeros in the decimal portion ONLY are significant.
Why does a ball eventually stop bouncing? Write a three- four sentence explaination
Answers
Answer:
The ball will stop bouncing because where the starting point of the ball dropping is the most potential energy it will have. The energy decrease will cause it to bounce lower and lower by each bounce. The ball's lowered energy will cause it to stop bouncing.
1.the particles in a_are packed very closely together. 2.the_in a liquid are loosely together. they can flow. 3.in a gas the_between the particles are bigger. if u re genius then answer this question class 4
Answers
Answer:
1. Solid
2. Particles.
3. Space.
Explanation:
1. The particles in a solid are packed very closely together. This is the most reason why solid particles have definite shapes of volume. This closeness is as a result of strong intermolecular forces force that exist between the particles. Hence, they can not flow but only vibrates about their mean position. Solids can not be compressed because of the strong intermolecular forces between their particles.
2. The particles of liquid are loosely together. In liquid, the particles are loosely packed and free to move about to certain degree. This is so because the intermolecular force between the particles are not as strong as those within the solid particles. Hence liquid has no definite shape but they have definite volume. They only assume the shape of the container they are poured into. Liquid can no be compressed..
3. In a gas, the space between the particles are bigger. This is so because the intermolecular forces between the particles are negligible i.e very small and so, the gas particles are free to move about and only restricted by the wall of the container they poured into. This negligible intermolecular forces are the reason why gas has no definite shapes and no volume. They can be compressed to fill a particular container.
N2(g)+3H2(g)->2NH3(g), ΔH=-92.40kJ 1. How many grams of H2 are needed to involve 150.9kJ of heat? 2. How many moles of NH3 were produced in the process?
Answers
1. To solve for the grams of H2 needed, we need to use the given ΔH value to calculate the amount of moles of N2 that reacted. From the balanced chemical equation, we know that for every 3 moles of H2 that reacts, 1 mole of N2 reacts. Therefore, we can use the mole ratio to convert the moles of N2 to moles of H2 and then use the molar mass of H2 to convert to grams.
First, we need to calculate the moles of N2 that reacted to produce 150.9kJ of heat:
ΔH = -92.40 kJ/mol N2
150.9 kJ = (1 mol N2 / -92.40 kJ) x (-150.9 kJ)
mol N2 = 1.63 mol
Using the mole ratio from the balanced chemical equation:
1 mol N2 : 3 mol H2
We can calculate the moles of H2 needed:3 mol H2 = 1 mol N2
3 mol H2 = 1.63 mol N2
mol H2 = 0.543 mol
Finally, we can convert moles of H2 to grams:
mol H2 = 0.543 mol
molar mass of H2 = 2.02 g/mol
grams of H2 = (0.543 mol) x (2.02 g/mol)
grams of H2 = 1.10 g
Therefore, 1.10 grams of H2 are needed to involve 150.9kJ of heat.
2. To solve for the moles of NH3 produced, we can use the same mole ratio from the balanced chemical equation:
1 mol N2 : 2 mol NH3
From the moles of N2 that reacted calculated in part 1, we can calculate the moles of NH3 produced:
1 mol N2 = 2 mol NH3
1 mol N2 = 1.63 mol N2
mol NH3 = (2 mol NH3 / 1 mol N2) x (1.63 mol N2)
mol NH3 = 3.26 mol
Therefore, 3.26 moles of NH3 were produced in the process.
For more questions on: moles
https://brainly.com/question/15356425
#SPJ11
There are 3 reactions of Calcium Carbonate, CaCO₃, that can be formed in this particular problem:
Reaction 1: Calculate the ΔH₁
Reaction 2: ΔH₂ = -635.1 kJ
Reaction 3: ΔH₃ = 178.3 kJ

Answers
ΔH₁ = ΔH₂ - ΔH₃
ΔH₁ = -635.1-(178.3) KJ
ΔH₁ = -813.4 KJ
A buffered solution _______. Select the correct answer below: fails to keep hydronium and hydroxide ion concentrations nearly constant when strong acids or bases are added. maintains a constant or nearly constant pH when small amounts of strong acids or bases are added. acts to keep the hydroxide ion concentration nearly constant. acts to keep the hydronium ion concentration nearly constant.
Answers
The correct option for the given question about Buffer Solution is Option B) which is maintains a constant or nearly constant pH when small amounts of strong acids or bases are added.
What is a Buffer Solution?When a little quantity of acid or base is diluted or added, the buffer solution undergoes very slight variations in its hydrogen ion concentration (pH). pH may be maintained in buffer solutions, which are mixtures of a weak acid and its conjugate base or a weak base and its conjugate acid.Acidic and alkaline buffers are the two main groups into which buffer solutions are commonly categorized.A weak acid and its salt are combined with a strong base to create an acidic buffer, which has an acidic pH.A weak base, its salt, and a strong acid are combined to create an alkaline buffer, which has a basic pH.
Thus we conclude that when weak acids or bases are supplied in small amounts, the pH remains steady or almost constant.
Learn more about Buffer Solution here:
https://brainly.com/question/26416276
#SPJ2
The periodic table of elements have a vertical___ that tell you the number of__ __ and horizontal __ that tell you the number of__ __
-electrons,electron,groups,valance,shells,periods
Answers
The periodic table of elements has vertical groups that tell you the number of electrons and horizontal periods that tell you the number of valance shells. The chemical elements are arranged in the periodic table according to their atomic numbers, starting with hydrogen, which has the lowest atomic number, and moving up to uranium, which has the greatest atomic number.
Due to their comparable chemical behavior, the periodic table's vertical columns are referred to as groups or families. The number of valence electrons and chemical characteristics is the same for all members of a family of elements. Periods refer to the horizontal rows on the periodic table.
The contemporary periodic table has 18 vertical columns and 7 horizontal periods.
To know more about periodic table, visit;
https://brainly.com/question/29766008
#SPJ1
Which best explains why the trend in noble gas boiling points increases down the group?A) increasing dispersion interactions B) increasing dipole-dipole interactions C) increasing ion-dipole interactions D) increasing hydrogen bonding interactions E) increasing ion-ion interactions
Answers
Answer:
A) increasing dispersion interactions
Explanation:
Polarizability allows gases containing atoms or nonpolar molecules (for example, to condense. In these gases, the most important kind of interaction produces dispersion forces, attractive forces that arise as a result of temporary dipoles induced in atoms or molecules.
Dispersion forces, which are also called London forces, usually increase with molar mass because molecules with larger molar mass tend to have more electrons, and dispersion forces increase in strength with the number of electrons. Furthermore, larger molar mass often means a bigger atom whose electron distribution is more easily disturbed because the outer electrons are less tightly held by the nuclei.
Because the noble gases are all nonpolar molecules, the only attractive intermolecular forces present are the dispersion forces.
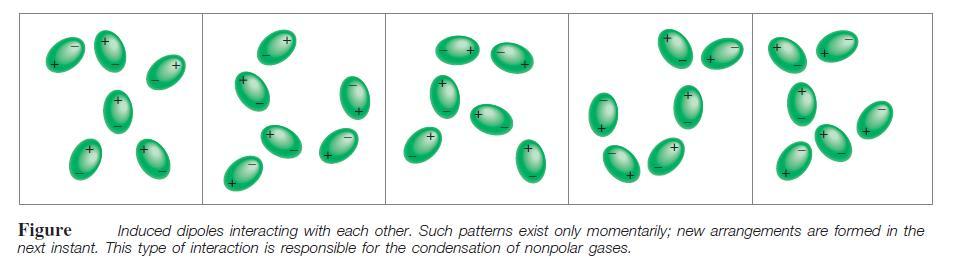
The boiling point of the noble gas increases on moving down the group because of the increase in the dispersion force. Thus, option A is correct.
The noble gas has been consisted of the fully complete octet of the atom and has been less reactive in nature.
On moving down the group, there has been an increase in the number of shells of the atom with the increase in the atomic mass. The bigger molecules tend to have loosely bonded outermost electrons.
In noble gases, the force acting upon the molecule has been the Dispersion force. Since, the noble gas has a complete octet, the energy for removing the electrons and changing the state increase with the increase in the molecular mass.
The only force acting on the noble gases has been the dispersion force, Thus with an increase in the dispersion force, the boiling point increases. Thus, option A is correct.
For more information about the boiling point of the noble gases, refer to the link:
https://brainly.com/question/2289319
Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) Calculate the volume N2O5 that must decompose completely to produce 9.64 L nitrogen dioxide.
Answers
The volume of \(N_2O_5\) needed to produce 9.64 L of \(NO_2\) is 4.97 L, calculated using stoichiometry and the ideal gas equation.
The given chemical equation is \(2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)\) .The volume of \(N_2O_5\) that decomposes completely to form 9.64 L of \(NO_2\) is to be calculated. For this, we can use the concept of stoichiometry. Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a balanced chemical equation.To calculate the volume of \(N_2O_5\) that is needed to produce 9.64 L of \(NO_2\), we will first determine the number of moles of NO2 produced in the reaction. For this, we can use the ideal gas equation, PV = nRT. Here, we have the volume of NO2 and we can assume the pressure and temperature to be constant. Thus, we have PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature. Substituting the given values in the ideal gas equation, we get,n = PV/RT = (1 atm × 9.64 L)/(0.0821 L atm K-1 mol-1 × 300 K) = 0.404 molFrom the chemical equation, we see that 2 moles of \(N_2O_5\) give 4 moles of \(NO_2\). Thus, 0.404 mol of \(NO_2\) must have been produced from (0.404/2) = 0.202 mol of \(N_2O_5\). Using the ideal gas equation, we can also find the volume of 0.202 mol of \(N_2O_5\) at the given conditions. Thus, V = nRT/P = (0.202 mol × 0.0821 L atm K-1 mol-1 × 300 K)/1 atm = 4.97 L. Thus, the volume of \(N_2O_5\) that must decompose completely to produce 9.64 L nitrogen dioxide is 4.97 L.For more questions on stoichiometry
https://brainly.com/question/14935523
#SPJ8
SCl6, K2S, ClO2, N2O, NaO2 Which of the following compounds are formed by ionic bonding?
Answers
Answer:
• SCl6 [ Sulphur hexachloride ]
• Na2O [ Sodium oxide ]
Or:
• Na2O2 [ Sodium peroxide ]
Explanation:
→ Ionic bonding or electrovalent bonding is the type of bonding between a metal and a non metal, where a metal loses its Outermost electrons to the non metal in order to gain stability.
\(.\)

What is the mass of 6.02 x 1024 molecules of the compound HCl?
Answers
Answer:
First, we need to determine the molar mass of HCl.
The molar mass of HCl = the mass of hydrogen (1.008 g/mol) + the mass of chlorine (35.45 g/mol) = 36.45 g/mol.
Next, we can use Avogadro's number (6.02 x 10^23 molecules/mol) to convert the number of molecules to moles:
6.02 x 10^24 molecules / 6.02 x 10^23 molecules/mol = 10 moles
Finally, we can use the molar mass to convert moles to grams:
10 moles x 36.45 g/mol = 364.5 grams
Therefore, the mass of 6.02 x 10^24 molecules of HCl is 364.5 grams.
:. It means 1 mole of Hcl
:. To find the mass of HcL
no of moles = mass/ molar mass
To get the molar mass of HCL {H=1 CL=35.5}
:. H+CL = 1+ 35.5 =36.5
So we have our molar mass and number of moles now
Then we input it in the eqn
1=x/36.5
X= 36.5g of HCl
convert 7.54 x 10^-8 m to nanometers
Answers
7.54 *\(10^8\) meters is 75.4 nanometers.
To convert 7.54 * \(10^8\) meters to nanometers, you can multiply the value by \(10^9\)
as, \(10^9\)nanometers = 1 meter.
7.54 * \(10^8\) m * \(10^9\) = 7.54 x \(10^1\) nm
Therefore, 7.54 *\(10^8\) meters is equal to 75.4 nanometers.
learn more about conversion:
https://brainly.com/question/13076223
To convert 7.54 x 10^-8 meters to nanometers, you multiply 7.54 x 10^-8 by 1 x 10^9 to get 75.4 nanometers.
Explanation:To convert meters to nanometers, you need to know that 1 meter is equivalent to 1 x 109 nanometers. Therefore, if you were to convert 7.54 x 10-8 m to nanometers, you would multiply 7.54 x 10-8 by 1 x 109.
Here's how you'd do it: 7.54 x 10-8 m * 1 x 109 nm/m = 75.4 nm. So, 7.54 x 10-8 meters is equivalent to 75.4 nanometers.
Learn more about Unit Conversion here:https://brainly.com/question/32030244
#SPJ2
During chemical reaction 7.55gKI and 9.06g were allowed to react. How many grams of excess reagent are left over after the reaction is complete. Reaction: Pb(NO3)2(s) + 2KCI(s) > 2KNO3(s) + PbI(s)
Answers
Answer: 7.45 g of \(Pb(NO_3)_2\) excess reagent are left over after the reaction is complete.
Explanation:
To calculate the number of moles, we use the equation:
\(\text{Number of moles}=\frac{\text{Given mass}}{\text{Molar mass}}\)
a) \({\text{Number of moles of} KI}=\frac{7.55g}{166g/mol}=0.045moles\)
b) \({\text{Number of moles of} Pb(NO_3)_2}=\frac{9.06g}{331.2g/mol}=0.027moles\)
The balanced chemical reaction is :
\(Pb(NO_3)_2(s)+2KI(s)\rightarrow 2KNO_3(s)+PbI(s)\)
According to stoichiometry :
2 moles of \(KI\) require = 1 mole of \(Pb(NO_3)_2\)
Thus 0.045 moles of \(KI\) will require=\(\frac{1}{2}\times 0.045=0.0225moles\) of \(Pb(NO_3)_2\)
Thus \(KI\) is the limiting reagent as it limits the formation of product and \(Pb(NO_3)_2\) is the excess reagent as (0.045-0.0225) = 0.0225 moles are left
Mass of \(Pb(NO_3)_2=moles\times {\text {Molar mass}}=0.0225moles\times 331.2g/mol=7.45g\)
Thus 7.45 g of \(Pb(NO_3)_2\) of excess reagent are left over after the reaction is complete.
How many total atoms are in 0.550 g of P2O5?
Answers
Answer:
Total atoms in 0.550g of =1.631x1022
Explanation:
answer is above
Describe how crystals change if they form in large amounts of space vs limited space.
Answers
Crystals form when magma cools because the solution is super-saturated with certain minerals. Because the crystals do not have much time to form if the magma cools quickly, they are very small. The crystals have enough time to grow and become large if the magma cools slowly.
What causes big crystals to form?When the solution becomes supersaturated, which means there is too much salt dissolved in the water, crystals form. Crystals form from the extra salt (or other material). To obtain a supersaturated solution, either cool the solution or allow some of the water to evaporate.
The size of the crystals is affected by the solubility of the compound in the solvent used for recrystallization, the number of nucleation sites, mechanical agitation of the system, and time during crystal growth.
Thus, the crystals do not have much time to form if the magma cools quickly, they are very small.
To learn more about the crystals, follow the link;
https://brainly.com/question/13008800
#SPJ9
distinguish between solutions and colloids.give an example of each
Answers
A solution is a combination of two or more substances in which the molecules are evenly distributed throughout. A colloid is a combination of two or more substances in which the molecules are not evenly distributed.
A solution is a kind of homogeneous mixture composed of two or more substances. The term aqueous solution is termed when one of the solvents is water. In such a mixture, solute and solvent are the two components in the solution.
The mixing process of a solution happens at a macroscopic scale via the interchange of particles between the solvent and solute.
A colloid is a substance that is composed of particles that are too small to be seen with a microscope, but are large enough so that they do not pass through a semipermeable membrane. An example of a colloid is milk.
To know more about homogeneous solutions, click below:
https://brainly.com/question/13490059
#SPJ9
Calculate the wavelength and frequency of an emitted photon of gamma radiation that has energy of 2.93 × 109 J/mol
Answers
Answer:
Explanation: Calculate the wavelength and frequency of an emitted photon of gamma radiation that has energy of 2.93 × 109 J/mol
Which refers to any disturbance that carries energy from one place to another?
Answers
Answer:
A wave
Explanation:
A wave is a disturbance that carries energy from one place to another. Matter is not carried with the wave but instead a wave can move through matter.
Answer:
A Wave
Explanation: