1. 54 mol HCl and 3. 6 mol NaOH react according to the equation
HCl + NaOH → NaCl + H2O
If the limiting reactant is HCl, determine the amount of excess reactant that remains. Answer in units of mol
Answers
To determine the amount of excess reactant that remains, we first need to identify the limiting reactant in the given reaction. The limiting reactant is the one that is completely consumed and determines the maximum amount of product that can be formed.
We can use the stoichiometry of the balanced chemical equation to compare the number of moles of each reactant to see which one is limiting.
From the balanced equation, we can see that the stoichiometric ratio between HCl and NaOH is 1:1. This means that for every 1 mole of HCl reacted, 1 mole of NaOH is consumed.
Given:
HCl: 54 mol
NaOH: 3.6 mol
Since the stoichiometric ratio is 1:1, we can determine that 3.6 moles of NaOH would require 3.6 moles of HCl for complete reaction. However, we have 54 moles of HCl, which is significantly more than required. Therefore, HCl is the excess reactant.
To calculate the amount of excess HCl that remains, we subtract the moles of NaOH (which is the limiting reactant) from the initial moles of HCl:
Excess HCl = Initial moles of HCl - Moles of NaOH
Excess HCl = 54 mol - 3.6 mol
Excess HCl = 50.4 mol
Therefore, 50.4 moles of HCl remain as excess reactant.
to know more about stoichiometry click this link
brainly.com/question/28780091
#SPJ11
To determine the amount of excess reactant that remains, we first need to identify the limiting reactant in the given reaction. The limiting reactant is the one that is completely consumed and determines the maximum amount of product that can be formed.
We can use the stoichiometry of the balanced chemical equation to compare the number of moles of each reactant to see which one is limiting.
From the balanced equation, we can see that the stoichiometric ratio between HCl and NaOH is 1:1. This means that for every 1 mole of HCl reacted, 1 mole of NaOH is consumed.
Given:
HCl: 54 mol
NaOH: 3.6 mol
Since the stoichiometric ratio is 1:1, we can determine that 3.6 moles of NaOH would require 3.6 moles of HCl for complete reaction. However, we have 54 moles of HCl, which is significantly more than required. Therefore, HCl is the excess reactant.
To calculate the amount of excess HCl that remains, we subtract the moles of NaOH (which is the limiting reactant) from the initial moles of HCl:
Excess HCl = Initial moles of HCl - Moles of NaOH
Excess HCl = 54 mol - 3.6 mol
Excess HCl = 50.4 mol
Therefore, 50.4 moles of HCl remain as excess reactant.
to know more about stoichiometry click this link
brainly.com/question/28780091
#SPJ11
Related Questions
how much force is required to accelerate an 1,800 kg truck at 3 m/s/s
Answers
Explanation: divide 3 m/s/s by 1,800 kg to get 600 N
Answer:
3 ÷1,800
= 600 N
Thus the force needed is 600N
pleae help me I'm so confused
What is the velocity of a person that runs 40 km in 100 hours?
Answers
Answer:
0.4 km/h velocity is also the direction their going, in this case the person, that I'll name billy bob joe, is going in so yeh
Explanation:
give me brainliest?
Is C6H1206 an element or compound
Answers
C6H1206 is a compound (of glucose).
A car is moving with the velocity of 20m/s. After 5 seconds it's velocity becomes 50m/s. Find its acceleration.
Answers
Answer:
6 m/s^2
Explanation:
a= (v2 - v1)/t
= (50-20)/5
=6
Answer:
6 m/s²
Explanation:
Given, Initial velocity (u) = 20m/s
Final velocity (v) = 50m/s
time taken (t) = 5 sec
Now,
Acceleration = v-u/t
= 50-20/5
= 30/5
= 6 m/s²
Which statement describes the Richter scale?
A. It cannot account for fault movement during an earthquake.
B. It measures large earthquakes far from the seismograph.
C. It estimates the total energy released from an earthquake.
D. It increases in magnitude as amount of damage increases.
Answers
Answer:
D. It increases in magnitude as amount of damage increases.
Explanation:
I took the test and chose A got it wrong and it said it was D
Answer:
D
Explanation:
I need help ASAP pls

Answers
Which of the following best describes Avogadro's number?*
A) An equation that can be used to calculate how many atoms are in one mole of
anything
B) A variable that represents the value of the atomic mass relative to one mole of an
element
C) A constant that states how many atoms/molecules are in one mole of anything
D)An expression in scientific notation that represents the average atomic mass of an
element
Answers
Answer:
C) A constant that states how many atoms/molecules are in one mole of anything
Explanation:
Avogadro's number like the name suggests is a constant that states how many atoms/molecules are in one mole of anything.

When heated, KClO3 decomposes into KCl and O2. 2KClO3⟶2KCl+3O2 If this reaction produced 13.2 g KCl, how many grams of O2 were produced?
Answers
Answer:
8.5gm O2 produced
Explanation:
When heated, KClO3 decomposes into KCl and O2. 2KClO3⟶2KCl+3O2 If this reaction produced 13.2 g KCl, how many grams of O2 were produced?
for every 2 moles of KCl produced, 3 moles of O2 are produced
Mol weight of KCl =39+35.5=74.5gm
13.2 gm KCl = 13.2/74.5 = 0.177 moles
this will make (3/2) X 0.177 = 0.2655 moles of O2
O2 mol wt is 32 0.2655 X32 = 8.5gm O2 produced
When this equation is balanced,
2C4H10 (g) + __O2(g) —> ___CO2 (g) + __H2O (g)
What is the coefficient of oxygen, O2?
Answers
Answer:
The answer is 11Explanation:
2C4H10 (g) + 11O2(g) —> 8CO2 (g) + 6H2O (g)
Hope this helps you
Hope this helps you
Answer:
2C4H10 + 2O2 ---> 8CO2 + 10H2O
Explanation:
What is another name for a row on the periodic table?
Answer choices :
Group
Period
Family
Transition Metals
Answers
Which of the following statements best describes a nebula?
Answers
Answer:
a cloud of adult stars that floats through space where protostars are born.
Explanation:
hope this helped:)
brainliest for the brains:)
In the reaction of nitrogen with hydrogen to produce ammonia, NH3, how many moles of ammonia would be produced from 1.0 mol of hydrogen and excess nitrogen?
N2 + 3 H2 --> 2 NH3
0.33 mol
0.67 mol
2.0 mol
1.3 mol
Answers
According to the equation;
3 mol of hydrogen will produce 2mol of ammonia.
1 mol of hydrogen _____________________?
= 2 × 1/3
= 0.67 mol
Answer:
0.67 mol
Explanation:
Given data:
Number of moles of ammonia produced = ?
Number of moles of hydrogen = 1.0 mol
Amount of nitrogen = excess
Solution:
Chemical equation:
N₂ + 3H₂ → 2NH₃
Now we will compare the moles of ammonia with hydrogen.
H₂ : NH₃
3 : 2
1.0 : 2/3×1.0 = 0.67
Thus, from 1.0 mole of hydrogen 0.67 moles of ammonia will produced.
Can someone pls help me with this pls

Answers
Answer:
I would say element
Explanation:
because it is made of two different types of atoms chemically bonded together!
I'm sorry if wrong!
In the TCA cycle, ____ oxidation(s) use(s) NAD as the oxidizing agent while ____ oxidation(s) use(s) FAD as the oxidizing agent.
Answers
Tricarboxylic acid cycle often occur in stages. In the TCA cycle, 3 oxidation(s) use(s) NAD as the oxidizing agent while 1 oxidation(s) use(s) FAD as the oxidizing agent.
Tricarboxylic acid cycle commonly called TCA cycle is also regarded as the Krebs cycle and citric acid cycle. It is known to be the second stage of cellular respiration.It consist of a three-stage process where living cells uses organic fuel molecules in the presence of oxygen to take-up energy that is needed for growth and division.
See full question below
In the TCA cycle, ____ oxidation(s) use(s) NAD + as the oxidizing agent while ____ oxidation(s) use(s) FAD as the oxidizing agent.
a. 0; 4
b. 1; 3
c. 2; 2
d. 3; 1
e. 4; 0
Learn more about TCA cycle from
https://brainly.com/question/8075492
4) The principle of ________ states that the physical, chemical, and biological processes at work shaping the Earth today have also operated in the geologic past.
A) catastrophism
B) plate tectonics
C) plutonism
D) Uniformitarianism
Answers
The principle of option D. Uniformitarianism states that the physical, chemical, and biological processes at work shaping the Earth today have also operated in the
Option D. Uniformitarianism is the principle stating that the physical, chemical, and biological processes at work shaping the Earth today have also operated in the geologic past. It is based on the idea that the present is the key to the past. In other words, the same natural laws that operate in the universe today have been operating since the beginning of time.
James Hutton was the first to propose this principle in the late 18th century. He suggested that the Earth was shaped by slow-acting geological forces such as erosion, sedimentation, and uplift over long periods of time. He believed that the same processes were still happening today and that they had operated in the past.
This principle is an important concept in geology because it allows scientists to interpret the Earth's history based on the processes that they observe today. By understanding how these processes work and how they have changed over time, scientists can reconstruct the history of the Earth and its environments.
Uniformitarianism has been tested and proven through many observations and experiments. For example, the study of sedimentary rocks has shown that they were formed in the past through the same processes that are observed today, such as deposition of sediment by water, wind, or ice.
Similarly, the study of volcanoes has shown that they are formed by the same processes as today, such as the movement of magma from deep within the Earth.
In conclusion, Uniformitarianism is the principle that allows us to interpret the Earth's history by observing the processes that shape it today. It is a fundamental concept in geology and has been tested and proven through many observations and experiments.
To know more about Uniformitarianism here
https://brainly.com/question/1324266
#SPJ11
What's the name of the part of cells that sense molecules and other cells?
Answers
Which of the following is NOT a reason why chemical bonds are important? *
a:Bonds are used to make new substances.
b:Building and breaking bonds are part of the energy cycle.
c:Bonds create new elements.
d:Chemical bonds sustain life.
Answers
Answer:
the answer would be B :)
The correct answer is; Bonds create new elements
Chemical bonds are formed when two or more atoms are joined together to form a compound.
Chemical compounds are more stable than the respective elements from which they are formed.
Chemical bonds do not create new elements.
Learn more; https://brainly.com/question/6071754
A Bronsted-Lowry acid is defined as a substance that ________. acts as a proton donor decreases [H ] when placed in H2O increases [OH-] when placed in H2O increases Ka when placed in H2O acts as a proton acceptor
Answers
A Bronsted-Lowry base is defined as a substance that C. acts as a proton acceptor.
This concept focuses on the transfer of protons (H+) in a chemical reaction. When a Brønsted-Lowry base is placed in water (H2O), it accepts a proton from a water molecule, forming a hydroxide ion (OH-) and increasing the concentration of hydroxide ions in the solution. This process distinguishes the Brønsted-Lowry base from a Brønsted-Lowry acid, which acts as a proton donor.
In a typical acid-base reaction, a Brønsted-Lowry base interacts with a Brønsted-Lowry acid, resulting in the transfer of a proton from the acid to the base. This process generates a conjugate acid and a conjugate base. The conjugate acid is the product formed when the base gains a proton, while the conjugate base results from the acid losing a proton. This proton transfer helps maintain the balance of H+ and OH- ions in the solution.
In summary, the key characteristic of a Brønsted-Lowry base is its ability to act as a proton acceptor, which increase in the concentration of hydroxide ions (OH-) when placed in water. This definition provides a framework for understanding the behavior of bases in acid-base reactions and their role in maintaining the equilibrium of H+ and OH- ions in a solution. Therefore the correct option C
Know more about proton acceptor here:
https://brainly.com/question/31326493
#SPJ11
Fill in the SI unit for each of the following measurements. 1: Time: ___ 2. Length:___ 3. Mass:___ 4. Temperature___
Answers
the si unit for time is second
the si unir for length is meter
the si unit for mass is kilogram
the si unit for temperature is kelvin
1: Time: second 2. Length: meter 3. Mass: kilogram 4. Temperature kelvin are the SI units of measurements.
What do you mean by SI unit of measurements?
It is unit of the standard of reference which is chosen to measure any type of physical quantity.
SI Units of Measurements is the system which stands for the international system of unit.
The SI, uses standard symbols, units and abbreviations, developed by the Bureau International.
The SI unit of mass is the kilogram, length is a meter, time is second, temperature is kelvin, electric current is ampere, amount of substance is mole, luminous intensity is candela.
SI unit has a huge amount of application universally in both technical and scientific research to avoid the confusion.
This system is important as it helps the entire world to understand the measurements in one set of unit systems.
Learn more about SI unit, here:
https://brainly.com/question/12750330
#SPJ2
which of the compounds can undergo racemization at the alpha carbon?
Answers
Compounds that can undergo racemization at the alpha carbon are chiral molecules with a stereocenter at the alpha carbon.
Racemization refers to the conversion of a chiral compound into a mixture of its enantiomers. This process can occur through a variety of mechanisms, such as acid-catalyzed epimerization or nucleophilic substitution. However, compounds that do not have a chiral alpha carbon, such as propanol, cannot undergo racemization.
These compounds have an asymmetric alpha carbon atom, which is bonded to four different groups, resulting in two non-superimposable mirror images called enantiomers. Typically, racemization occurs when the alpha carbon is attached to a carbonyl group, as in amino acids and alpha-hydroxy acids. Through various chemical reactions, these compounds can convert between their enantiomers, leading to a racemic mixture of equal amounts of both forms.
Learn more about alpha carbon here:
https://brainly.com/question/31665878
#SPJ11
why a mixture of magnesium hydroxide and water is known as milk of magnesia
Answers
because of its milk-like appearance. ... Since the dissociation of this small amount of dissolved magnesium hydroxide is complete, magnesium hydroxide is considered a strong electrolyte. Its low solubility makes it a weak base.
A mixture of hydrogen and iodine, each at 55 KPa and hydrogen iodide at 78 KPa was introduced into a container heated at 783 K. At this temperature K= 46 for the following reaction: H2 (g)+l2 (g) = HI (g) a.Q< K; HI will decompose into Hź and l2 b.Q>K; HI will be formed c.Q K; HI will decompose into H2 and l2
Answers
at the given temperature, HI will decompose into H2 and I2.
Given that the following reaction has an equilibrium constant value of
K = 46 at 783K: H2 (g) + l2 (g) = HI (g).
Initial pressures were given to be 55kPa for both hydrogen and iodine and 78kPa for hydrogen iodide which is at equilibrium. In this problem, Qp is the reaction quotient for pressures at the given instant. Qp has the same expression as Kp, but with initial pressures instead of equilibrium pressures.
Qp = p(HI) / [p(H2) . p(I2)] = 78 / [55 . 55] = 0.0241
K is the equilibrium constant and Q is the reaction quotient.Q is less than K. This implies that the reaction quotient will increase to match the equilibrium constant.
As a result, the reaction will shift forward to produce more HI. Thus, at the given temperature, HI will decompose into H2 and I2.
learn more about equilibrium constant here
https://brainly.com/question/3159758
#SPJ11
Balance the chemical equation. Values may be used more than once.
Ga + H2SO4 ⇒ Gaz(SO4)3 + H2
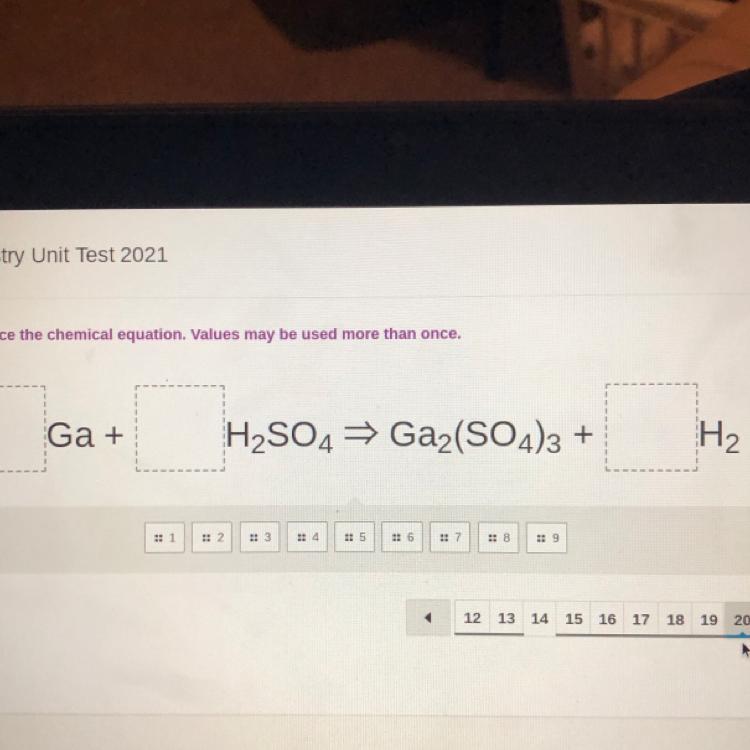
Answers
\(\\ \sf\longmapsto Ga+H_2SO_4\longrightarrow Ga_2(SO_4)_3+H_2\)
Balanced equation:-
\(\\ \sf\longmapsto 2Ga+3H_2SO_4\longrightarrow Ga_2(SO_4)_3+3H_2\)
On reactant side
Ga=2H=6SO_4=3On products side
Ga=2H=6SO_4=3Hence balanced
how is it that life on earth has changed over million of years yet the Earth has not?
actually environmental science own words please need it now asap guys please help me it’s due today.
Answers
4. Jon found a fossil and was curious to find out the age of the
fossil. When the lab tested it they found the ratio to be 12.5%
C-14 and 87.5% N-14. How old was the fossil?
Answers
The age of the fossil that is found is estimated to be 17,190 years old.
What is the age of the fossil?The age of the fossil will be calculated using the formula for radioactive decay which is \(N(t) = N₀ * e^{-λt}\)
For carbon-14, the decay constant is 0.00012097 per year.
Assuming initial amount of carbon-14 in the fossil was equal to the amount in the atmosphere which is 1 part per trillion \(10^{-12}\), we will calculate the age of the fossil as \(0.125 = 10^{-12} * e^{-0.00012097t}\)
Taking natural logarithm
t = (ln 0.125) / (-0.00012097)
t = - 2.07944154168 / -0.00012097
t = 17189.7292029
t = 17,190 years.
Read more about fossil
brainly.com/question/11224681
#SPJ4
How many moles of hydroxide (OH-) are in 25. 0 mL of 1. 00 M NaOH?
Answers
0.025 moles of hydroxide ions (OH-) are there in 25.0 mL of 1.00 M NaOH.
We may use the following formula to calculate the number of moles of hydroxide ions (OH-) in 25.0 mL of 1.00 M NaOH:
moles = concentration x volume
First, we need to convert the volume from milliliters to liters, which can be done by dividing by 1000:
25.0 mL = 25.0/1000 = 0.025 L
plugging in the values:
moles of NaOH = concentration x volume = 1.00 mol/L x 0.025 L = 0.025 mol
Since NaOH dissociates completely in water to produce one mole of hydroxide ions for every mole of NaOH, we have:
moles of OH- = 0.025 mol
Therefore, there are 0.025 moles of hydroxide ions (OH-) in 25.0 mL of 1.00 M NaOH.
For more such questions on hydroxide ions, click on:
https://brainly.com/question/28464162
#SPJ11
Explain why Nitrogen -17 is unstable
Answers
if you add 5 ml of 0.5 m naoh solution to 20 ml each of buffer b (with a ph of 4.03) and buffer c, which buffers ph would change the least?
Answers
If you add 5 ml of 0.5 M NaOH solution to 20 ml each of buffer B (with a pH of 4.03) and buffer C, the buffer whose pH will change the least is buffer B.
What is a buffer?
A buffer is a solution that resists changes in pH when acid or alkali is added. A buffer solution is a solution that contains a weak acid and its corresponding weak base or a weak base and its corresponding weak acid.
The Henderson-Hasselbalch equation can be used to calculate the pH of a buffer solution before and after adding a strong base or acid.
The equation is pH = pKa + log ([A-]/[HA])
where:pKa is the dissociation constant for the acid[A-] is the conjugate base of the acid when a command (such as NaOH) is added to a buffer solution, the base reacts with the weak acid to form the conjugate base of the acid and water. The addition of the conjugate base of the acid causes the pH of the solution to rising.
When an acid (such as HCl) is added to a buffer solution, the acid reacts with the weak base to form the conjugate acid of the base and water. The addition of the conjugate acid of the base causes the pH of the solution to decrease. Based on the above equation, pH change will be minimum in Buffer B. Therefore, the buffer whose pH will change the least is buffer B.
#SPJ11
to learn more about buffer : https://brainly.com/question/24262133
glyceraldehyde 3-phosphate dehydrogenase transfers a hydride ion (two electrons and a proton) from its substrate. which molecule is the recipient of the proton and two electrons during this transfer?
Answers
Glyceraldehyde 3-phosphate dehydrogenase transfers a hydride ion (two electrons and a proton) from its substrate. The molecule that is the recipient of the proton and two electrons during this transfer is nicotinamide adenine dinucleotide (NAD+).
Explanation:
Nicotinamide adenine dinucleotide (NAD+) is a coenzyme that accepts hydride ions in cellular respiration. It is one of the important coenzymes involved in the electron transport chain of cellular respiration.
Glyceraldehyde 3-phosphate dehydrogenase is one of the enzymes involved in the metabolic pathway known as glycolysis, which takes place in the cytoplasm of the cell.
During glycolysis, glyceraldehyde 3-phosphate dehydrogenase catalyzes the reaction that converts glyceraldehyde 3-phosphate into 1,3-bisphosphoglycerate. During this reaction, it also transfers a hydride ion from its substrate, which is accepted by NAD+ to form NADH.
Hence, NAD+ molecule is the recipient of the proton and two electrons during this transfer.
To know more about NAD+, refer here:
https://brainly.com/question/15427146#
#SPJ11
What do we mean if I say a chemical equation is balanced?
Answers
When we say a chemical equation is balanced, we mean that the law of conservation of mass is valid.
A chemical equation is said to be balanced if the number of atoms in each element on both the reactant and product sides of the equation is equal. This means that the law of mass conservation is followed, and the total number of atoms of each element is conserved during the chemical reaction.
Adjusting the coefficients of the reactants and products to reach the same amount of atoms of each element on both sides is what balancing a chemical equation entails.
To know more about balanced chemical equation, visit,
https://brainly.com/question/11904811
#SPJ1