1.) a copper penny has a mass of 3.1 g and a volume of 0.35 cm^3. what is the dencity of the copper penny?
2.) A block of wood has the dimensions 7.0 cm x 10.0 cm x 5.0 cm. What is the
volume of the block of wood?
3.) A graduated cylinder has a volume of 50.0 ml of water. After a rubber cork is
placed in the graduated cylinder, the volume of the water rises to 53.0 ml. If
the mass of the rubber cork is 4.3 g, what is its density?
4.) Calculate the volume of a book with a density of 9.9 g/cm3 and a mass of 58.5 g.
5.) Find the mass of iron if its density is 6.7 g/ml and it has a volume of 5 ml.
Answers
1.)
density is found by \(\rho=\frac{m}{V}\)
\(\rho=\frac{3.1}{0.35}\\\\ \rho=8.857 g/cm^3\)
2.)
Volume is \(lwh\)
7*10*5
V=350\(cm^3\)
3.)
using the density formula
\(\rho=\frac{m}{V}\)
\(\rho=\frac{4.3}{3}\)
\(\rho=1.43g/ml\)
4.)
rearrange the density formula for V
\(V=\frac{m}{\rho}\)
\(V=\frac{58.5}{9.9}\\\\ V=5.909cm^3\)
5.)
rearrange the density formula for \(m\)
\(m=\rho V\\\\m=(6.7)(5)\\\\m=33.5g\)
Related Questions
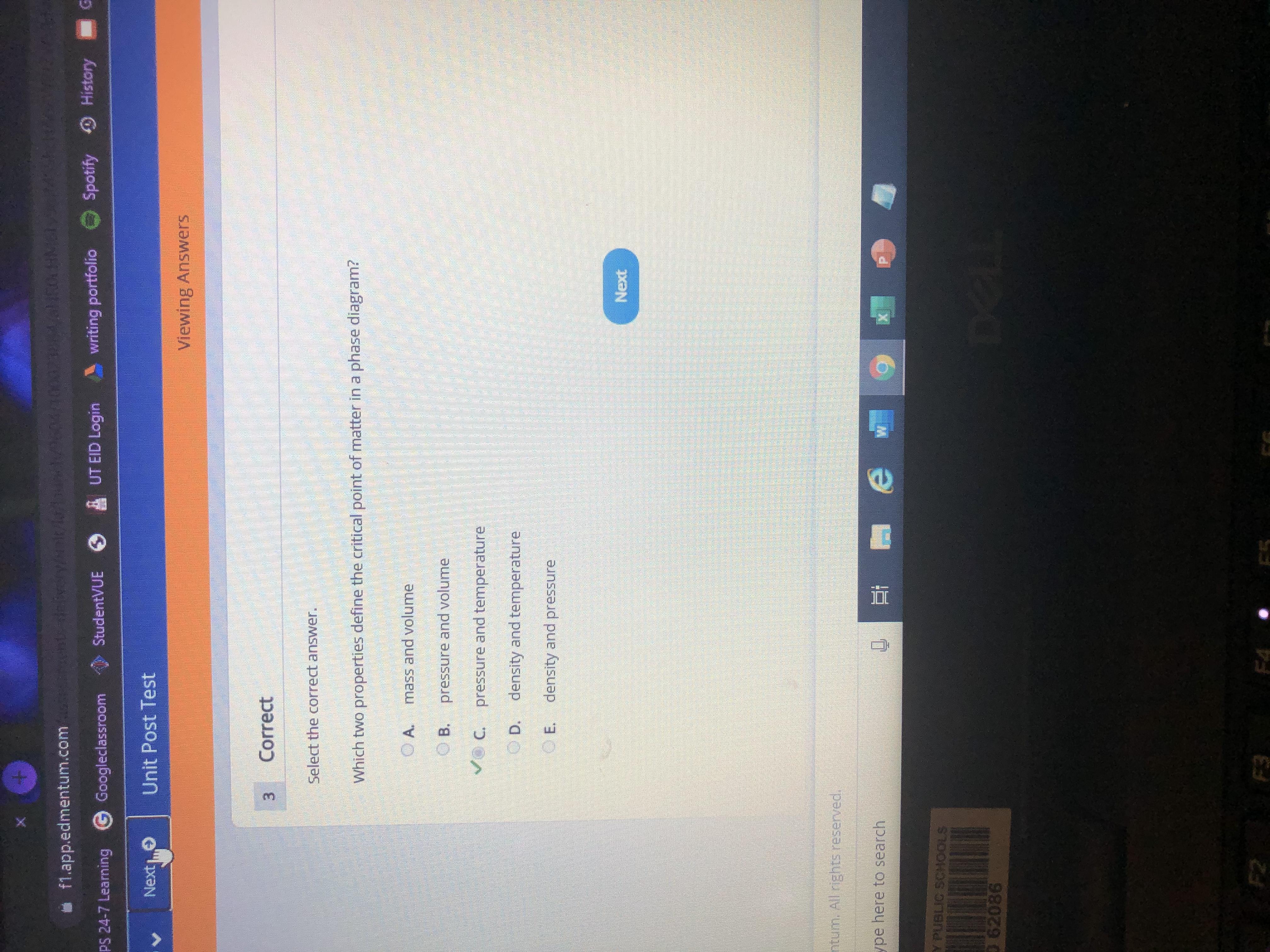
Which two properties define the critical point of matter in a phase diagram?

Answers
Answer:
C: pressure and temp
Explanation:
What pattern can you determine about the location of the volcanoes and the earthquake zones? Also explain why the earthquake zones are long lines on the globe. Write 3 to 5 sentences.
Answers
Pattern: Volcanoes and earthquake zones are often found in close proximity to each other, with earthquake zones appearing as long lines on the globe.
The pattern can be attributed to the tectonic plate boundaries. Volcanoes are typically located along plate boundaries where there is subduction or collision of tectonic plates. These areas experience intense geological activity, leading to volcanic eruptions. Earthquake zones, represented by long lines on the globe, coincide with plate boundaries as well. The lines correspond to the locations where plates interact, resulting in significant stress and release of energy, leading to seismic activity. The long lines represent the interconnectedness of multiple earthquake-prone areas along the plate boundaries, forming seismic belts or zones. Therefore, the close proximity of volcanoes and earthquake zones can be explained by their shared association with tectonic plate boundaries and the geologically active regions that result from plate interactions.
Learn more about tectonic here:
https://brainly.com/question/2816983
#SPJ11
what is the solution to the equation below rounded to the correct number of significant figures? 4700 L - 281.4 = _____ L
Answers
Answer:4400
I went over this problem multiple times and I finally found the soultion
The concept of significant figures are mainly used by scientist and engineer to know the significance of digits in a measurement. Therefore, 4400L is the solution to the equation rounded to the correct number of significant figures.
What is significant figures?Significant figures are the figures that indicate the degree of accuracy of a value. It tells about the precision of a value. It gives an idea about the digits that are necessary to indicate the experimental value.
Rules for counting significant figures are:
Number between 1 to 9 is always significant
Zeroes after a number has got no significance
Zeroes before a number has got no significance
Zeroes between number has got significance
4700 L - 281.4 =4400L
Therefore, 4400L is the solution to the equation rounded to the correct number of significant figures.
To learn more about Significant figures, here:
https://brainly.com/question/12656148?
#SPJ2
How would an increased level of acetyl-CoA be expected to affect the pyruvate dehydrogenase reaction
Answers
Answer:
The pyruvate dehydrogenase kinase enzyme activity would increase, resulting in an inhibition of pyruvate dehydrogenase activity. ... An in vitro study shows that isocitrate dehydrogenase is activated in the citrate cycle.
Explanation:
Consider a reaction whose rate constant is 3. 4 m-1s-1 at 600k and 31. 0 m-1s-1 at 750k. Find the activation energy (in kj/mol) of the reaction. Express your answer to 2 decimal places
Answers
The activation energy of the reaction is approximately 71.46 kJ/mol, rounded to 2 decimal places.
To find the activation energy (Ea) of the reaction, we can use the Arrhenius equation, which relates the rate constant (k) to the temperature (T) and activation energy. The Arrhenius equation is given by:
k = A * e^(-Ea/RT)
Where:
k is the rate constant
A is the pre-exponential factor (frequency factor)
Ea is the activation energy
R is the gas constant (8.314 J/(mol·K))
T is the temperature in Kelvin
We have two sets of data:
At 600 K, k1 = 3.4 m^(-1)s^(-1)
At 750 K, k2 = 31.0 m^(-1)s^(-1)
Taking the natural logarithm (ln) of both sides of the Arrhenius equation, we can rearrange the equation to solve for the activation energy:
ln(k) = ln(A) - (Ea/RT)
We can create two equations using the given data:
ln(k1) = ln(A) - (Ea/(R * 600))
ln(k2) = ln(A) - (Ea/(R * 750))
Subtracting the second equation from the first eliminates the ln(A) term:
ln(k1) - ln(k2) = (Ea/R) * ((1/600) - (1/750))
Simplifying further:
ln(k1/k2) = (Ea/R) * ((750 - 600) / (600 * 750))
Now we can solve for Ea:
Ea = (R * (ln(k1/k2))) / ((750 - 600) / (600 * 750))
Using the given values and the appropriate units:
Ea = (8.314 J/(mol·K) * ln(3.4/31.0)) / ((750 - 600) / (600 * 750))
Converting the units from J to kJ:
Ea = (8.314 × 10^(-3) kJ/(mol·K) * ln(3.4/31.0)) / ((750 - 600) / (600 * 750))
Ea ≈ 71.46 kJ/mol
Therefore, the activation energy of the reaction is approximately 71.46 kJ/mol, rounded to 2 decimal places.
learn more about activation energy here
https://brainly.com/question/28384644
#SPJ11
4. What coefficients do you need to balance the following equation?
CH4 + O2 + CO2 + H2O
O 1, 2, 1, 2
O 2, 1, 1, 1
O 1, 1, 1, 1
O 2, 1, 2, 2
Answers
Answer:
option first is the right answer
Calculate the change in entropy of 3 moles of liquid water if you heat it from 5Ëšc to 95Ëšc. The molar heat capacity of liquid water is 75. 38 j mol-1 k-1. Please report your answer one point past the decimal with the unit j/k.
Answers
The change in entropy is equal to 63.4 J/K.
What is entropy?Entropy is a measureable physical characteristic and a scientific notion that is frequently connected to a condition of disorder, unpredictability, or uncertainty. Entropy can be thought of as a rough indicator of the quality of energy, with lower entropy indicating higher quality. Lower entropy energy is structured for storage (the efficient library). Energy is chaotically stored in the random-pile library, which has a high entropy. The second law of thermodynamics states that the total entropy of a system may only ever increase or remain constant during spontaneous processes.
What is the best example of entropy?Ice melting is the ideal illustration of entropy. The individual molecules are arranged and fixed as ice. As the ice melts, the molecules become disordered because they are now able to migrate. The molecules are subsequently liberated to travel independently through space as the water is heated to become a gas.
Briefing:
One of the thermodynamic properties is entropy. A system's change in entropy is influenced by changes in temperature, volume, or pressure.
The following formula is used to determine the change in entropy:
ΔS = n Cv lnT₂//T₁ + nR ln V₂/V₁
where,
n = number of moles
Cv = heat capacity
T = temperature
R = gas constant
V = volume
Given:
Number of moles (n) = 3
Temperature (T₁) = 5° C = 278 K
Temperature (T₂) = 95° C = 368 K
Molar heat capacity Cv = 75.38\ J/mol • K
Now, we can calculate the entropy change as follows:
ΔS =nCvlnT₂/T₁
ΔS = 3 × 75.38 × ln(368/278)
ΔS = 226.14 × ln13.24
ΔS = 63.4 J/K
As a result, the entropy change is 63.4 J/K.
To know more about Entropy visit:
https://brainly.com/question/13999732
#SPJ4
the products of the electron transport chain include group of answer choices h2o. glucose. o2. nadh.
Answers
Answer:hat are the products of electron transport chain?
The end products of electron transport are NAD+, FAD, water and protons. The protons end up outside the mitochondrial matrix because they are pumped across the cristal membrane using the free energy of electron transport.
Explanation:
Given the standard enthalpy changes for the following two reactions
Given the standard enthalpy changes for the following two reactions:
(1) 2C(s) + 2H2(g)C2H4(g)...... ΔH° = 52.3 kJ
(2) 2C(s) + 3H2(g)C2H6(g)......ΔH° = -84.7 kJ
what is the standard enthalpy change for the reaction:
(3) C2H4(g) + H2(g)C2H6(g)......ΔH° = ?
Answers
The standard enthalpy change for reaction (3) is 117.1 kJ.
The standard enthalpy change for reaction (3) can be calculated by using the enthalpy changes of reactions (1) and (2) and applying Hess's Law.
To do this, we need to manipulate the given equations so that the desired reaction (3) can be obtained.
First, we reverse reaction (1) to get the formation of C2H4(g) from C2H6(g):
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
Next, we multiply reaction (2) by 2 and reverse it to obtain 2 moles of C2H6(g) reacting to form 3 moles of H2(g):
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
Now, we add the two modified equations together:
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
When adding these equations, the C2H6(g) on the left side cancels out with the C2H6(g) on the right side, leaving us with the desired reaction (3):
C2H4(g) + H2(g)C2H6(g) ΔH° = -52.3 kJ + 169.4 kJ = 117.1 kJ
Learn more about standard enthalpy here :-
https://brainly.com/question/28303513
#SPJ11
H 0
:p A
=0.40,p B
=0.40, and p C
=0.20 H a
: The population proportions are not p A
=0.40,p B
=0.40, and p C
=0.20. sample of size 200 yielded 140 in category A, 20 in category B, and 40 in category C. Use α=0.01 and test to see whether the proportions are as stated in H 0
. (a) Use the p-value approach. Find the value of the test statistic. Find the p-value. (Round your answer to four decimal places.) p-value = State your conclusion. Reject H 0
. We conclude that the proportions differ from 0.40,0.40, and 0.20. Do not reject H 0
. We cannot conclude that the proportions are equal to 0.40,0.40, and 0.20. Do not reject H 0
. We cannot conclude that the proportions differ from 0.40, 0.40, and 0.20. Reject H 0
. We conclude that the proportions are equal to 0.40,0.40, and 0.20. (b) Repeat the test using the critical value approach. Find the value of the test statistic. State the critical values for the rejection rule. (If the test is one-tailed, enter NONE for the unused tail. Round your answers to three decimal places.) test statistic ≤ test statistic ≥ State your conclusion. Reject H 0
. We conclude that the proportions differ from 0.40,0.40, and 0.20. Do not reject H 0
. We cannot conclude that the proportions differ from 0.40, 0.40, and 0.20. Do not reject H 0
. We cannot conclude that the proportions are equal to 0.40,0.40, and 0.20. Reject H 0
. We conclude that the proportions are equal to 0.40,0.40, and 0.20.
Answers
The test results indicate that we reject the null hypothesis (H0) and conclude that the proportions are not equal to 0.40, 0.40, and 0.20.
What is the value of the test statistic and the p-value for the given hypothesis test?To test the hypothesis, we can use the p-value approach. The test statistic for comparing proportions is the chi-square statistic (χ²). In this case, we have three categories (A, B, and C) and their respective observed frequencies (140, 20, and 40) in a sample of size 200.
The expected frequencies under the null hypothesis can be calculated by multiplying the sample size by the hypothesized proportions. Thus, the expected frequencies are 80 for category A, 80 for category B, and 40 for category C.
Using these observed and expected frequencies, we can calculate the chi-square test statistic:
χ² = Σ[(observed - expected)² / expected]
After calculating the test statistic, we can find the p-value associated with it using the chi-square distribution with degrees of freedom equal to the number of categories minus 1.
Comparing the p-value to the significance level (α = 0.01), if the p-value is less than α, we reject the null hypothesis; otherwise, we fail to reject it.
Learn more about null hypothesis
brainly.com/question/30821298
#SPJ11
Describe the formation of ionic bonds between elements from Group I and Group VII, including the use of dot-and-cross diagrams
Answers
Explanation:
In a physical change the nature of the substance, the particles of which it is composed and the numbers of particles remain unchanged. In a chemical change the properties of the new substances are different from the original, the particles are different and the number of particles can change
The formation of ionic bonds between elements from Group I (alkali metals) and Group VII (halogens) occurs through a transfer of electrons. Alkali metals have one valence electron in their outermost shell, while halogens require one more electron to complete their outermost shell and achieve a stable electron configuration.
Let's take the example of sodium (Na) from Group I and chlorine (Cl) from Group VII to illustrate the formation of an ionic bond. Sodium has one valence electron, while chlorine requires one electron to complete its outer shell.
In a dot-and-cross diagram, sodium is represented by the symbol Na, with a dot next to it representing its single valence electron. Chlorine is represented by the symbol Cl, with seven dots around it representing its seven valence electrons.
To form an ionic bond, sodium will transfer its single valence electron to chlorine. This results in sodium losing one electron to become a positively charged sodium ion (Na+), as it now has one less electron than protons. Chlorine, on the other hand, gains the electron from sodium, resulting in a negatively charged chloride ion (Cl-), as it now has one more electron than protons.
In the dot-and-cross diagram, the electron transfer is represented by an arrow from sodium to chlorine, indicating the movement of the electron. The final configuration shows the sodium ion (Na+) with no dots around it, indicating the loss of its valence electron, and the chloride ion (Cl-) with eight dots around it, representing the complete octet in its outermost shell.
The resulting sodium ion and chloride ion are held together by the strong electrostatic attraction between the oppositely charged ions. This attraction forms an ionic bond, creating an ionic compound known as sodium chloride (NaCl), commonly known as table salt.
This process of electron transfer and formation of ionic bonds occurs between elements from Group I and Group VII, leading to the creation of stable compounds with full outer shells for both elements.\(\huge{\mathcal{\colorbox{black}{\textcolor{lime}{\textsf{I hope this helps !}}}}}\)
♥️ \(\large{\textcolor{red}{\underline{\texttt{SUMIT ROY (:}}}}\)
An unevenly heated plate has temperature T(x,y) in∘C at the point (x,y). If T(2,1)=140, and T_x(2,1)=16, and T_y (2,1)=−15. estimate the temperature at the point (2.03,0.96). T(2.03,0.96)≈
Answers
Using the given partial derivatives at (2,1), the estimated temperature at (2.03, 0.96) is approximately 139.5°C based on first-order approximation.
To estimate the temperature at the point (2.03, 0.96), we can use a first-order approximation based on the given information. The first step is to use the partial derivatives of temperature, T_x and T_y, at the point (2,1). These derivatives provide the rate of change of temperature with respect to the x and y coordinates.
Given T_x(2,1) = 16 and T_y(2,1) = -15, we can use these values to estimate the change in temperature for small changes in x and y around the point (2,1). Since we want to estimate the temperature at (2.03, 0.96), which is a small change from (2,1), we can approximate the change in temperature as follows:
ΔT = T_x(2,1) * Δx + T_y(2,1) * Δy
Here, Δx = 2.03 - 2 = 0.03 and Δy = 0.96 - 1 = -0.04 (as we are subtracting the coordinates of (2,1) from (2.03, 0.96)).
Substituting the values, we have:
ΔT = 16 * 0.03 + (-15) * (-0.04)
= 0.48 + 0.6
= 1.08
Since T(2,1) = 140, we can estimate T(2.03, 0.96) by adding the change in temperature to the initial temperature:
T(2.03, 0.96) ≈ 140 + 1.08
= 141.08
Rounding this to the nearest tenth, the estimated temperature at (2.03, 0.96) is approximately 139.5°C.
Learn more about estimated temperature
brainly.com/question/26251133
#SPJ11
The outer shell of this element was determined as illustrated here.
It must be a _____.
Answers
Matter and your body-how are they
interrelated?
Answers
Answer:
Matter has mass and takes up space, everything has matte, your body takes up space. Matter refuels the body. You can also so your body produces matter as well
Some viruses attack cells by attaching to their outer covering entering and taking over their genetic machinery.viruses are able to invade cells after first attaching to their
Answers
If a 200 g piece of aluminum has a density of 5.0 g/cm^3. what is its volume?
Answers
Answer:
Volume=mass in g /density
Answer: 40cm^3 or 40ml
Explanation:
\(Density=\frac{mass}{volume}\)
so 5=200/V
V=200/5
V=40cm^3
Compare and contrast qualitative and quantitative data.
Answers
Answer: Qualitative data typically consists of words while quantitative data consists of numbers.
Explanation:
Substance P is soluble in water but insoluble in propanone.
Substance Q is insoluble in water but soluble in propanone.
Substance R is insoluble in both water and propanone.
Describe how to obtain a pure dry sample of each of P, Q, and R from a mixture of P+Q+R.
Answers
35x³-25x4y³/5x²y²
A botanist measures a plant growth at 3cm over a two week period. The information she gathers is called.
Answers
Answer:
The correct answer is quantitative data.
Explanation:
The value of data in the form of numbers of counts where each set of data exhibits a specific numerical value associated with it is termed as quantitative data. This information refers to any quantifiable knowledge, which can be used for statistical analysis and mathematical calculations so that decisions of real-life can be taken based on the mathematical outcomes. The quantitative data is used to find the solutions of the queries like how often, how much, or how many.
In the given case, a botanist measured the growth of the plant for two weeks, and the outcome came in the form of numerical value. Thus, the knowledge she collected is known as quantitative data.
If a hypothetical future Earth has increased the amount of radiation emitted to 604 W/m, calculate the hypothetical global surface temperature in degrees Kelvin and then in Celsius
Answers
Answer:
Explanation:
For amount of radiation from a hot body there is Stefan's Boltzman's law which is given below .
E = e σ T⁴
E is amount emitted , e is emissivity , σ is stefan's constant = 5.67 X 10⁻⁸ W / m² . for earth e =.85 approx
E = .85 x 5.67 X 10⁻⁸ x T⁴
604 = .85 x 5.67 X 10⁻⁸ x T⁴
604 = 4.82 x 10⁻⁸ T⁴
T⁴ = 125.31 x 10⁸
T = 3.3457 x 10²
= 334.57K
Temperature in Celsius = 334.57 - 273
= 61.57 Celsius .
Which best describes nitrogen fixation?
Answers
Answer:
Nitrogen fixation is the process of converting nitrogen to a usable form.
According to the department of transportation, hazardous materials are defined as a megerial that can pose an unreasonable risk to
Answers
According to the Department of Transportation (DOT), hazardous materials are defined as materials that can pose an unreasonable risk to health, safety, or property during transportation.
These materials can include explosives, flammable gases and liquids, toxic substances, radioactive materials, and more. The DOT has specific regulations in place for the transportation of these materials to ensure that they are handled and transported safely to minimize the risk of accidents or harm.
These regulations include:
Requirements for labeling, packaging, and transporting hazardous materials, as well as training for individuals involved in the transportation process.It is important to follow these regulations to prevent accidents and protect the health and safety of those involved in the transportation of hazardous materials.
Learn more about hazardous materials at: https://brainly.com/question/29232461
#SPJ11
What Is It?
Imagine you find a bowl filled
with clear liquid in your kitchen. List three liquids it might be. Describe how you could use the liquid's physical and chemical properties to determine which of the three it is.
Answers
Answer:
physical
Explanation: water can change from solid to liquid to a gas
Which pair of compounds represents one arrhenius acid and one arrhenius base?.
Answers
Explanation:
use the diagram above to understand it

what two biological processes might influence ph in coastal areas?
Answers
Two biological processes that might influence pH in coastal areas are photosynthesis and respiration.
Photosynthesis is the process by which plants and other photosynthetic organisms use sunlight to convert carbon dioxide into oxygen and organic compounds. During this process, they take up carbon dioxide from the surrounding water, which can cause a decrease in pH.
Respiration is the process by which organisms release energy from organic compounds, such as glucose, in order to power their cellular functions. This process produces carbon dioxide, which can increase the acidity of the surrounding water and lead to a decrease in pH.
These two biological processes are important factors to consider when studying pH levels in coastal areas. Understanding how they affect the surrounding environment can help scientists better predict and manage changes in pH caused by natural or human-induced disturbances.
learn more about photosynthesis
https://brainly.com/question/19160081
#SPJ11
Vt=227. 5 mL 0. 150 M barium hydroxide used to titrate acid Va= 50. 0 mL chloric acid
Find: Molarity of chloric acid 2HClO3 + 1Ba(OH)2 → 2H2O + 1Ba(ClO3)2
Answers
The molarity of chloric acid during titration is found to be 068M.
The given reaction is 2HClO₃ + Ba(OH)₂ → 2H₂O + Ba(ClO₃)₂
In the reaction, 0.150M Barium hydroxide is titrated using chloric acid.
The volume of chloric acid is 50mL and the volume of Barium hydroxide is 227.5mL.
Now, we know from the reaction,
2 moles of Chloric acid is titrated with one mole of barium hydroxide.
Now, we can write,
Molarity x volume of Chloric acid = Molarity x volume of barium hydroxide.
Now, putting the values,
0.150 x 227.5 = 50 x molarity of chloric acid
molarity of chloric acid = 0.68M.
So, the molarity of Chloric acid is 0.68M.
To know more about molarity, visit,
https://brainly.com/question/14469428
#SPJ4
what is likely to happen to a liquid mixture of water and rubbing alcohol in an open flask as temperature is increased while pressure stays the same?
Answers
When the mixture of water and rubbing alcohol are put in an open flask and the temperature is increased while keeping the pressure same the rubbing alcohol will vaporize at a faster rate.
The mixture of water and rubbing alcohol is a homogeneous mixture.
When the temperature of the flask is increased in which the mixture of water and rubbing alcohol is put the kinetic energy of the molecule associated with the mixture is increased at a very higher rate.
As we are keeping the flask in a place of the region of atmospheric pressure so we can assume that the pressure will always remain the same.
As a result the rubbing alcohol starts to evaporate at a much higher rate even before reaching the boiling point.
To know more about temperature, visit,
https://brainly.com/question/24746268
#SPJ4
A 4.5-cm-diameter, 0.50-mm-thick spherical plastic shell holds carbon dioxide at 2.0 atm pressure and 25∘C. CO2 molecules diffuse out of the shell into the surrounding air, where the carbon dioxide concentration is essentially zero. The diffusion coefficient of carbon dioxide in the plastic is 2.5×10−12 m2/s What is the diffusion rate in molecules/s of carbon dioxide out of the shell? Express your answer in molecules per second. Part B If the rate from part A is maintained, how long in hours will it take for the carbon dioxide pressure to decrease to 1.0 atm ? The actual rate slows with time as the concentration difference decreases, but assuming a constant rate gives a reasonable estimate of how long the shell will contain the carbon dioxide. Express your answer in hours.
Answers
The diffusion rate of carbon dioxide out of the shell can be calculated using Fick's first law of diffusion, which states that the diffusion rate is proportional to the diffusion coefficient, the surface area, and the concentration difference.
First, we need to calculate the surface area of the shell:
The diameter of the shell is given as 4.5 cm, so the radius is half of that, which is 2.25 cm.
The surface area of a sphere is given by the formula A = 4πr^2.
Plugging in the radius, we get A = 4π(2.25 cm)^2 = 63.59 cm^2.
Next, we need to calculate the concentration difference:
The carbon dioxide concentration inside the shell is given as 2.0 atm, while the concentration outside the shell is essentially zero. The concentration difference is therefore 2.0 atm - 0 atm = 2.0 atm.
Now we can calculate the diffusion rate using the formula diffusion rate = diffusion coefficient * surface area * concentration difference. Plugging in the given values, we get diffusion rate = (2.5×10^(-12) m^2/s) * (63.59 cm^2) * (2.0 atm) = 3.18×10^(-9) cm^3·atm/s.
To convert this to molecules per second, we need to use Avogadro's number, which is 6.022×10^23 molecules/mol. Since carbon dioxide has a molar mass of approximately 44 g/mol, we can convert the diffusion rate to molecules per second by multiplying it by Avogadro's number and dividing by the molar mass of carbon dioxide. The molar mass of carbon dioxide is 44 g/mol = 44000 mg/mol.
diffusion rate (in molecules/s) = (3.18×10^(-9) cm^3·atm/s) * (6.022×10^23 molecules/mol) / (44000 mg/mol) = 4.34×10^14 molecules/s.
So, the diffusion rate of carbon dioxide out of the shell is 4.34×10^14 molecules/s.
For Part B, we can use the diffusion rate from Part A to calculate the time it takes for the carbon dioxide pressure to decrease to 1.0 atm.
The initial pressure is 2.0 atm and the final pressure is 1.0 atm.
Since the rate is constant, we can use the formula time = (final pressure - initial pressure) / diffusion rate.
Plugging in the values, we get time = (1.0 atm - 2.0 atm) / (4.34×10^14 molecules/s) = -2.3×10^(-15) s.
To convert this to hours, we divide by 3600 s/hour and take the absolute value to get time = |(-2.3×10^(-15) s) / (3600 s/hour)| = 6.4×10^(-19) hours.
So, it will take approximately 6.4×10^(-19) hours for the carbon dioxide pressure to decrease to 1.0 atm, assuming a constant diffusion rate.
Learn more about Fick's first law of diffusion:
https://brainly.com/question/33290149
#SPJ11
Will give brainliest

Answers
The electron configurations of two unknown elements x and y are shown. X: 1s2 2s2 2p6 Y: 1s2 2s2 2p6 3s2 3p6 Which statement is most likely correct about the two elements? A) They will react because X can give up two electrons B) They will react because X and Y can share two pairs of electrons to become stable C) They will not react because both have a complete outermost shell and are stable D) They will not react because both will give up one electron. to become stable.
Answers
Answer:
B) They will react because X and Y can share two pairs of electrons to become stable
Explanation:
The electron configurations of two elements x and y are given :
X: 1s2 2s2 2p6
Y: 1s2 2s2 2p6 3s2 3p6
The statement that is true for both the elements is that, they both will react as they both can share two pairs of electrons to become stable.
To become stable the outermost shell or p orbital should have 8 electrons, so element X can gain 2 atoms to become stable.
Element Y can also react as it can also share two atoms to fulfill its 3p orbital and will stable.
Hence, the correct option is "B".