1. Describe in your own words what friction is.
2. Describe how friction affected the results of your investigation.
3. Does an object travel farther on a smooth or slippery surface or on a rough surface? Why?
4. Propose another experiment that could be used to demonstrate that friction is a contact force.
10 points, please hurry.
Answers
Answer
There is much more friction on the rough surface that there is on the smooth surface.
Related Questions
Identify all the signs of a chemical change in the description below:
A clear liquid is placed in a beaker and heated. As the liquid is heated, bubbles form and, gradually, the liquid decreases in the container.
Answers
Answer:
When a substance undergoes changes in its chemical composition or combines with another substance to form a new substance is referred to as chemical change. In contrast, physical change is defined as the change in appearance or state of matter and not the chemical composition of the substance.
Explanation:
When a clear liquid is boiled or heated, the kinetic energy of the molecules increases, and the evaporation of the liquid takes place faster. For example, evaporation is a type of physical change, in which the liquid is heated to form bubbles or vapors. These vapors rise in the air and decrease the volume of the liquid. In this process, the liquid has only changed its state of matter (liquid to gas) and not the chemical composition. Thus, there is no chemical change taking place in the heating of water and only physical change is taking place.
Given 5.86 grams of NaN3, how many grams of Na are produced?
Answers
Answer:
what no entiendo nada jahag6
When 5.86 grams of NaN3, then 22.99 gram of Na are produced by using mole formula.
What is gram ?A thousandth of a kilo-gram in terms of mass in the metric system.
What is mole?A mole is a unit of measurement for atom amount. A mole of Iron atoms weighs heavier than just a mole of Carbon molecules because it is measured by the number of atoms present instead of by weight.
The reaction can be written as:
\(2 Na N_{3}\) → 2Na + \(3N_{3}\).
It can be seen that 2 mole of sodium azide give 3 mole of nitrogen molecule.
Calculate the moles of Na.
Moles of Na = 1 mol sodium azide × 2 mol of Na / 2 mol sodium azide = 1 mole of Na.
Calculate the mass of Na.
Mass of Na = 1 mol Na × 22.99 g Na / 1 mol Na = 22.99 gNa.
Therefore, the mass of Na will be 22.99 gram
To know more about mole.
https://brainly.com/question/26416088
#SPJ3
What would the approximate age of an
igneous rock that contains only 1/4 of its
original carbon-14 (half-life of carbon is
5700 years)
Answers
Explanation:
Carbon-14 has a half life of 5730 years, meaning that 5730 years after an organism dies, half of its carbon-14 atoms have decayed to nitrogen atoms. Similarly, 11460 years after an organism dies, only one quarter of its original carbon-14 atoms are still around.
What is the formula of A^4 +b^4
Answers
Answer:
A^4 +b^4
(a²)²+(b²)²
(a²+b²)²-2a²b²
or
(a²-b²)²+2a²b²
Where do nutrients(food and water) enter the body first?
O mouth
stomach
O esophagus
O rectum
Answers
Answer:
esophagus……………..
You first put food into your mouth
TRUE OR FALSE 15 POINTS
One benefit of asexual reproduction is that it is efficient and fast because one organism is involved. ______
Binary fission is the process of splitting into two identical organisms. ______
Budding involves a hydra budding an identical right off themselves. ______
Sexual reproduction involves the uniting of gametes. ______
Sperm and egg cells have 20 chromosomes. ______
One benefit of sexual reproduction is genetic diversity. ______
Genetic variety can exist in a population of grasshoppers due to asexual reproduction. ______
Answers
One benefit of asexual reproduction is that it is efficient and fast because one organism is involved. ⇒ True
Binary fission is the process of splitting into two identical organisms.⇒ True
What is reproduction ?The term reproduction is defined as the production of offsprings. There are two types of reproduction. 1. Sexual reproduction 2. Asexual reproduction.
Budding involves a hydra budding an identical right off themselves.⇒ False
Sexual reproduction involves the uniting of gametes. ⇒ True
Sperm and egg cells have 20 chromosomes.⇒ False
One benefit of sexual reproduction is genetic diversity.⇒ True
Genetic variety can exist in a population of grasshoppers due to asexual reproduction.⇒ False
Thus, One benefit of asexual reproduction is that it is efficient and fast because one organism is involved. ⇒ True
To learn more about the reproduction, follow the link;
https://brainly.com/question/14329745
#SPJ1
is this right someone please help asap photo attached above

Answers
CaCO3 (s) + 176 KJ à CaO (s) + CO (g)
A. 176 KJ are released in an endothermic reaction.
B. 176 KJ are released in an exothermic reaction
C. 176 KJ are absorbed in an exothermic reaction.
D. 176 KJ are absorbed in an endothermic reaction.
Answers
Answer:
its d
Explanation:
What kind of reaction is this? C5H8 + 7O2 --> 5CO2 + 4H2O
A. Decomposition
B. Combustion
C. Single replacement
D. Combination
Answers
Answer:
B. Combustion .
Explanation:
Hello.
In this case, since this reaction is started by pentyne and oxygen which are converted to carbon dioxide and water, we can infer this is a combustion reaction because it involves the burnt of a fuel, in this case the pentyne, by using oxygen in order to yield carbon dioxide and water, which are smaller molecules than the original pentyne.
It is important to notice that these reactions are very exothermic because they go to break the C-H bonds in the fuel which release a considerably high amount of energy.
Best regards!
Construct a conclusion using the claim, evidence and reasoning format to explain the typical properties observed by ionic and covalent compounds.
Answers
Answer:
The key to understanding why ionic and covalent compounds have different properties from each other is understanding what's going on with the electrons in a compound. Ionic bonds form when atoms have different electronegativity values from each other. When the electronegativity values are comparable, covalent bonds form.
But, what does this mean? Electronegativity is a measure of how easily an atom attracts bonding electrons. If two atoms attract electrons more or less equally, they share the electrons. Sharing electrons results in less polarity or inequality of charge distribution. In contrast, if one atom attracts bonding electrons more strongly than the other, the bond is polar.
Ionic compounds dissolve in polar solvents (like water), stack neatly on each other to form crystals, and require a lot of energy for their chemical bonds to break. Covalent compounds can be either polar or nonpolar, but they contain weaker bonds than ionic compounds because they are sharing electrons. So, their melting and boiling points are lower and they are softer.
I hope this helps have a great day :)
The main purpose of the muscular system is to ____
A. Lift heavy things
B. Allow for movement
C Allow for structure
D. Rid the body of unwanted waste
Answers
Answer: B. Allow for movement
Explanation: Because the muscular system is composed of specialized cells called muscle fibers. Their predominant function is contractibility. Muscles, attached to bones or internal organs and blood vessels, are responsible for movement. Nearly all movement in the body is the result of muscle contraction. Make sure to add me as a friend!!! <3 YW!!!
NEED HELP ASAP!!!!!!!!! The process labeled #6 in this picture is known as
A. endocytosis
B. exocytosis
C. passive transport
D. diffusion

Answers
Answer:
B - Exocytosis
Explanation:
Exocytosis is the process by which a large amount of molecules are released; thus it is a form of bulk transport. In exocytosis, membrane-bound secretory vesicles are carried to the cell membrane, and their contents are secreted into the extracellular environment.
F-Test is designed to indicate whether there is a significant difference between two methods based on their standard deviations. The following results were obtained during the Gravimetric and Volumetric determination of iron in sample A. Gravimetric: 13.0, 13.5, 13.3, and 12.9. 15.1, 13.3, 12.7, 12.6, and 13.1. From the following two sets of replicate analyses on the same sample, determine whether the variance of gravimetric differs significantly from that of the volumetric method.
Answers
By performing the calculations and comparing the F-value to the critical F-value, we can determine whether the variance of the Gravimetric method differs significantly from that of the Volumetric method in the analysis of iron in sample A.
To determine if there is a significant difference between the variances of the Gravimetric and Volumetric methods used to determine iron in sample A, we can employ the F-test, also known as Fisher's F-test or the variance ratio test. The F-test compares the ratio of the variances to assess if there is a statistically significant difference.
Let's denote the Gravimetric method as Group 1 and the Volumetric method as Group 2. The first step is to calculate the variances for each group.
For Group 1 (Gravimetric):
Variance_1 = [(13.0-13.1)^2 + (13.5-13.1)^2 + (13.3-13.1)^2 + (12.9-13.1)^2 + (15.1-13.1)^2 + (13.3-13.1)^2 + (12.7-13.1)^2 + (12.6-13.1)^2 + (13.1-13.1)^2] / (n-1)
For Group 2 (Volumetric):
Variance_2 = Calculate the variance in the same manner for the data obtained from the Volumetric method.
Once we have the variances, we can calculate the F-value using the formula:
F = Variance_1 / Variance_2
Next, we need to compare this F-value with the critical F-value at a chosen significance level (usually α = 0.05 or 0.01) and degrees of freedom for each group (df_1 and df_2).
If the calculated F-value is greater than the critical F-value, it indicates a significant difference between the variances of the two methods. Conversely, if the calculated F-value is less than the critical F-value, there is no significant difference in the variances.
for more such questions on variance
https://brainly.com/question/6261134
#SPJ8
How many grams of Na2CO3 are in 5.61mol of Na2CO3?
Answers
Answer:
Na2co3 moles to grams
Explanation:
You can view more details on each measurement unit: molecular weight of Na2CO3 or grams This compound is also known as Sodium Carbonate. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Na2CO3, or 105.98844 grams.
In the dehydration of hydrated salt the sample was slightle moist because of high humidity briefly explain how the moisture affects the percentage calculation
Answers
Answer:
identify and name the landforms in the following diagrams
Compounds produces their hydrated forms by reacting with water molecules. This water content will make their weight measurement slightly erroneous.
What is dehydration?Dehydration is the process of removing water from a compound. Dehydration can be done by drying the compounds in room temperature or by heating.
Some compounds mostly existing in the hydrated form such as copper sulphate, magnesium sulphate etc. They rapidly reacts with water and form their hydrated compounds such as hexahydrate, pentahydrated etc according to the number of water molecules attached .
Most of the compounds are existing in their anhydrous form and they can be easily weighed without the extra weight added by the water content absorbed.
If the compound is moisturized from air there may be extra weight of water when we measure its mass. Thus, they have to heated to be dehydrated to correct the weight measurements.
To find more about dehydration, refer the link below:
https://brainly.com/question/12261974
#SPJ2
A 2.9 kg model rocket accelerates at 15.3 m/s2 with a force of 44 N. Before launch, the model rocket was not moving. After the solid rocket engine ignited, hot gases were pushed out from the rocket engine nozzle and propelled the rocket toward the sky.
Which of Newton’s laws apply in this example?
Answers
Answer:
Newton's first and third law of Motion
Explanation:
The laws applying in the example Newton's first and third laws of Motion.
The first law states that any object at rest (ie. not moving) will stay at rest until it is forced to move by an external force. In this case, said force were the propulsion gases ignited.As the hot gases were pushed out from the engine nozzle, there was another force equal in magnitud but opposite in direction (as the gases went down, that force went upwards), said force is directly responsible for the rocket taking off. That is an example of the third law.Answer:
It Newtons first, second, and third laws
Explanation:
Oxidation state of the iodine (I) in IO3– and chlorine (Cl) in ClO–?
Answers
Answer: The oxidation state of chlorine (Cl) in ClO– is +1.
Explanation:
To determine the oxidation state of iodine (I) in IO3– and chlorine (Cl) in ClO–, we can use the oxidation state rules.
For IO3– (iodate ion):
The sum of the oxidation states for all atoms in a polyatomic ion equals the charge of the ion. In this case, the charge is -1.
Oxygen typically has an oxidation state of -2.
There are three oxygen atoms in the iodate ion.
Let x be the oxidation state of iodine (I). Then, we can write the equation:
x + 3(-2) = -1
x - 6 = -1
x = +5
The oxidation state of iodine (I) in IO3– is +5.
For ClO– (hypochlorite ion):
The sum of the oxidation states for all atoms in a polyatomic ion equals the charge of the ion. In this case, the charge is -1.
Oxygen typically has an oxidation state of -2.
Let y be the oxidation state of chlorine (Cl). Then, we can write the equation:
y + (-2) = -1
y - 2 = -1
y = +1
The oxidation state of chlorine (Cl) in ClO– is +1.
Which of the following is a covalent compound? *
CO2
OK20
O Naci
O MgCl2
Answers
Answer:
this question is badly formatted. CO2
Explanation:
the answer is CO2 because C and O are both anions/nonmetals. the other examples contain a cation/metal, so they're ionic compounds
Suppose you are asked to find the area of a rectangle that is 2.1- cm wide by 5.6- cm long. Your calculator answer would be 11.76 cm2 . Now suppose you are asked to enter the answer to two significant figures.
Answers
which is the answer for this
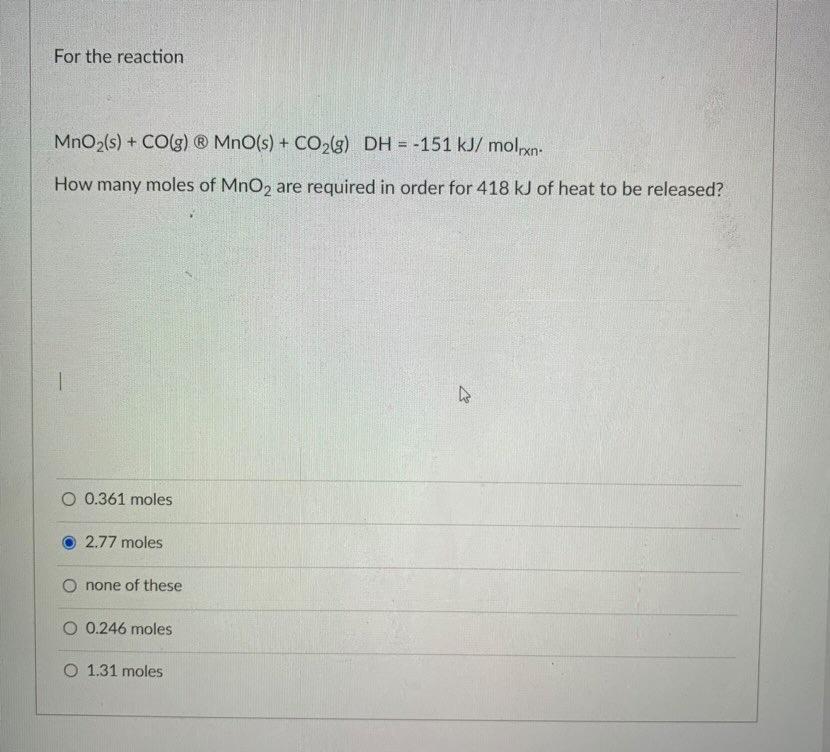
Answers
The answer is 2.767 moles of MnO2 for the given thermochemical equation is: MnO2 + CO → Mn + CO2, and the enthalpy change for this reaction is -151 kJ/mol..
What is 1 Joule?One joule (J) is the SI unit of energy, and it is defined as the amount of work done when a force of 1 newton (N) is applied over a distance of 1 meter (m). Alternatively, one joule can be defined as the amount of energy required to move an object with a force of 1 newton over a distance of 1 meter in the direction of the force.
From the thermochemical equation, we know that the enthalpy change for the reaction of 1 mole of MnO2 is -151 kJ/mol. Therefore, the heat released by the reaction of 1 mole of MnO2 is -151 kJ/mol.
Now we can take the reciprocal of the heat released by the reaction of 1 mole of MnO2, i.e.,
1 mole MnO2 / (-151 kJ/mol) = -0.006622 moles MnO2/kJ
This tells us that for every kilojoule of heat released by the reaction, we need 0.006622 moles of MnO2.
Calculate the number of moles of MnO2 needed to release 418 kJ of heat.
To do this, we can multiply the number of moles of MnO2 needed to release 1 kJ of heat by 418 kJ:
-0.006622 moles MnO2/kJ × 418 kJ = -2.767 moles MnO2
The negative sign in the answer means that we need a negative number of moles, which is not physically meaningful. Therefore, we can conclude that we need 2.767 moles of MnO2 to release 418 kJ of heat.
To know more about enthalpy, visit:
https://brainly.com/question/13996238
#SPJ1
17. Which of the following
hydrocarbon undergo addition
reaction:
С3Н6
С2Н6
ОООО
С3Н8
CH4
Answers
Answer:
С3Н6.
Explanation:
Hello!
In this case, since addition reactions imply that a radical or some radicals are added to the parent chain, we notice that only unsaturated hydrocarbons are able to undergo addition whereas saturated ones undergo substitution reactions as they already have all the carbon bonds bonded to leaving groups.
In such a way, we can rule out C2H6, C3H8 and CH4 as they are all alkanes; therefore, only С3Н6 is able to undergo an addition reaction due to the C=C which is able to lose one of those bonds and allow an incoming radical to get included into the parent chain.
Best regards!
3.
Examine the list of metals below.
I. Gold
II. Potassium
III. Chromium
IV. Zinc
V. Calcium
Which of these metals form more than one ion?
IV only
I and III
II and V
I, III, IV
Answers
Answer:
zinc forms more than one ion
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
Which cause of wildfires is least likely to lead to catastrophic results
O arson
Olightning strike
O controlled burn
O unattended campfire
Answers
The controlled burn cause of wildfires is least likely to lead to catastrophic results. Therefore, option (C) is correct.
What is controlled burning?A controlled burn is a fire set intentionally for purposes of forest management, prairie restoration, farming, or greenhouse gas. A controlled burn refers to the intentional burning of slash, fuels through burn piles.
Controlled burning can be done during the cooler months to reduce fuel buildup and reduce the likelihood of serious hotter fires. Controlled burning enhances the germination of desirable forest trees, and exposes soil mineral layers which expand seedling vitality.
Controlled burning is also useful in agriculture as it is often referred to as slash and burns. It is one component of shifting cultivation, as a section of field preparation for planting called field burning. This technique is utilized to clear the land of any existing crop residue and kill weeds and weed seeds.
Field burning is less expensive but because it forms smoke and air pollutants, its use is not common in agricultural areas.
Learn more about controlled burn, here:
https://brainly.com/question/28466648
#SPJ2
How does the speed of a sound wave change based on the density of the medium the wave passes through?
A. In a denser medium, the sound wave will speed up and then slow down.
B. Density does not affect how fast a sound wave will travel.
C. In a denser medium, the sound wave will travel slower.
D. In a denser medium, the sound wave will travel faster.
Answers
In a denser medium, the sound wave will travel slower.
What is sound?
Sound is a type of wave that is created by vibrations that propagate through a medium, such as air or water. These vibrations cause pressure variations in the medium, which our ears perceive as sound. The speed of sound wave propagation depends on the properties of the medium. In general, the denser the medium, the slower the speed of sound. For example, sound travels faster through solids than through liquids, and faster through liquids than through gases. The speed of sound is also affected by temperature and humidity, which can alter the density of the medium.To know more about sound, click the link given below:
https://brainly.com/question/29707602
#SPJ1
Draw the structures of the major and minor organic products formed when HBr reacts with 2,4,4-trimethyl-2-pentene in the presence of peroxides. When drawing hydrogen atoms on a carbon atom, either include all hydrogen atoms or none on that carbon atom, or your structure may be marked incorrect.
major product minor product
Answers
Answer:
Major product: 3-bromo-2,2,4-trimethylpentane
Minor product: 2-bromo-2,4,4-trimethylpentan
Explanation:
In this case, we will have an anti-markovnikov reaction. So, the Br (the nucleophile would be added in the least substituted carbon (in the double bond). On this molecule, the least substituted carbon of the double bond is the carbon in the left, therefore, the major product would be 3-bromo-2,2,4-trimethylpentane. With this in mind, the minor product would be the addition product in which the "Br" is added in the most substituted carbon of the double bond (carbon in the right) and we will have as a product 2-bromo-2,4,4-trimethylpentane. (See figure 1)
I hope it helps!

(01.01 LC)What is the body of scientific knowledge based on?
Guesses
Mysteries
Observations
Opinions
Answers
The body of scientific knowledge is based on different Observations (Option C).
What does observations mean in the scientific method?Observations in the scientific method are fundamental because it is the first step to raising scientific questions that may be explained through plausible hypotheses. Subsequently, hypotheses must be tested by experimental procedures.
In conclusion, the body of scientific knowledge is based on different Observations (Option C).
Learn more about observations in the scientific method here:
https://brainly.com/question/2505873
#SPJ1
Sodium sulfate forms several hydrates. A sample of one of these hydrates is heated until all the water is removed. What is the formula of the original hydrate if it loses 43% of its mass when heated?
Answers
Answer:
Na₂SO₄•(H₂O)₆.
Explanation:
The mass that is lost when the sample is heated is water.
Let's assume we have 100 g of the hydrate:
43 grams would be water (H₂O), while the rest (100-43=57) would be sodium sulfate anhydrous (Na₂SO₄).
We convert both those masses to moles, using their respective molar masses:
H₂O ⇒ 43 g ÷ 18 g/mol = 2.39 molNa₂SO₄ ⇒ 57 g ÷ 142.04 g/mol = 0.40 molWe can write those results as (Na₂SO₄)₀.₄₀•(H₂O)₂.₃₉. Now we just need to multiply those numbers so that they become integers.
If we multiply both coefficients by 5 we're left with (Na₂SO₄)₂•(H₂O)₁₂.
Simplify and thus the final answer is Na₂SO₄•(H₂O)₆.
BRAINLIEST TO CORRECT PLEASE

Answers
Answer:
A. last F first E second C thrid b foruth A five
Explanation:
21. Which energy level has the lowest amount of energy?
A. First
B. Second
C. Third
D. Fourth
Answers
Answer:
ground state
Explanation:
The lowest energy level of a system is called its ground state; higher energy levels are called excited states.
PLEASE MARK ME BRAINLIEST.\( {}^{} \)