1. Which five quantities a, b, c, d and e are required to balance the equation ... aTl2(CO3)3(s) + bHCl(aq) → cTlCl3(aq) + dH2O(l) + eCO2(g)
Answers
Answer:
\(a = 1\\ b = 6\\ c = 2 \\ d = 3\)
Explanation:
Greetings!!!
Have attached an image above look at it and if you have any questions tag it on comments
Hope it helps!!!


Related Questions
How are gases and liquids different from each other
Answers
Answer:
Gases, liquids, and solids are all made up of atoms, molecules, and/or ions, but the behaviors of these particles differ in the three phases. Gas is well separated with no regular arrangement. liquid are close together with no regular arrangement. solid are tightly packed, usually in a regular pattern.
Explanation:
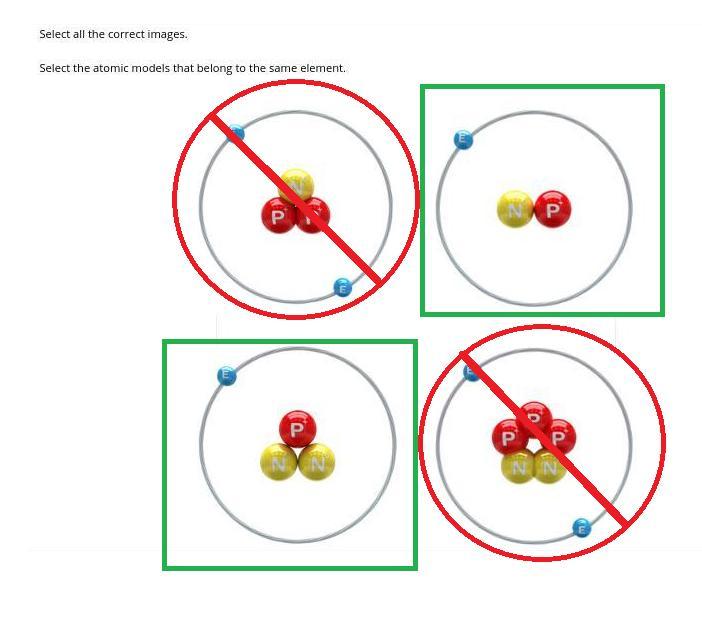
Can somebody please help me with this?

Answers
Answer: The atoms in the green boxes are the same element while the others in the red circles are of different elements.
Explanation:
Atoms of the same element MUST have the same number of protons. The number of neutrons may vary in the case of ISOTOPES (in which case the green labelled atoms are isotopes). Both red labelled atoms have a different number of protons from the other two.
Which of the following elements will NOT form
an ion with a -1 charge?
A)Lithium
B)Fluorine
C)Iodine
D)Chlorine
Answers
Answer:
Fluorine does not form an ion with a charge of 1+.
Using proper arrow formalism, write down a mechanism for the reaction between methyl pyruvate and benzylamine in order to produce a corresponding Schiff base. If acid is required in your mechanism, use HA as a generic acid.a. How would you expect the IR spectrum of methyl pyruvate to differ from the Schiff base product formed in Part 1? Be specific with the location of the different, distinct IR signals that would be present in each compound, and which functional groups they correspond to
Answers
a) Using arrow formalism, the mechanism for the reaction between methyl pyruvate and benzylamine to produce the corresponding Schiff base is as follows:
1) HA + benzylamine → HA-benzylamine
2) methyl pyruvate + HA-benzylamine → Schiff base + HA
b) The IR spectrum of methyl pyruvate is expected to differ from that of the Schiff base product formed in Part 1 in several ways. For methyl pyruvate, there will be a strong peak at 1722 cm-1 corresponding to the carbonyl functional group in the carboxylic acid, and at 1449 cm-1 for the alkene. For the Schiff base product, there will be a strong peak at 1632 cm-1 corresponding to the C=N double bond, and at 3450 cm-1 for the NH2 group.
Read more about the topic of the IR spectrum :
https://brainly.com/question/5951360
#SPJ11
PLS HELP ASAP
Which of these is an example of a chemical reaction that releases energy?
A.boiling water
B.burning wood
C.photosynthesis
D.melting ice cubes
Answers
Answer:
B) burning wood
Explanation:
im pretty sure it is burning wood
which reacts faster, rock salt or grains of salt? Why?
Answers
Answer:
salt
Explanation:
because it has a much larger volume ratio
What mass of K2SO4 must be added to 1.20 liters of water to produce a 1.50 M solution?
Answers
Answer:
313.2 g of \({K_{2} }S O_{4}\) must be added to 1.20 liters of water to produce a 1.50 molar solution.
Explanation:
What is molarity?
Molarity is a unit of concentration of a solution. It is defined by the number of moles of the solute that is present in one liter (1L) of the solution. It is denoted by M. Thus, molarity = \(\frac{Number of moles of the solute (n) }{Volume of the solution (V) (in L)}\)∴ The number of moles of solute = molarity x volume of the solution.According to the given question,
Molarity of the solution = 1.50 MThe volume of the solution = 1.20 LUnknown = Mass of \({K_{2} }S O_{4}\) required.Solution :
∴ Number of moles of solute, here, \({K_{2} }S O_{4}\)
= molarity x volume of the solution
= 1.20 x 1.50 = 1.8
∴ Mass of 1.8 moles of \({K_{2} }S O_{4}\) = 1.8 x molar mass of \({K_{2} }S O_{4}\)
Now the molar mass of \(K_{2} SO_{4}\)
= (Gram atomic mass of K x 2) + (Gram atomic mass of S) + (Gram atomic mass of O x 4)
= (39x2) + 32 + (16 x 4) g
= 174 g.
∴ Mass of 1.5 moles of \({K_{2} }S O_{4}\)
= 1.8 x molar mass of \({K_{2} }S O_{4}\)
= 1.8 x 174 g
= 313.2 g.
Thus, 313.2 g of \({K_{2} }S O_{4}\) must be added to 1.20 liters of water to produce a 1.50 M solution.
To know more about molarity, visit :
https://brainly.com/question/15406534
which of the following options correctly describe the oxidation of primary alcohols? select all that apply. multiple select question. primary alcohols require different oxidizing conditions than secondary alcohols. a carboxylic acid can be produced by oxidation of a primary alcohol. during oxidation, a primary alcohol will rearrange to produce a more substituted oxidation product. mild oxidizing conditions will result in an aldehyde product. harsher oxidizing conditions will produce a ketone from a primary alcohol.
Answers
The options that describe the oxidation of the primary alcohols is a carboxylic acid can be produced by oxidation of a primary alcohol. Mild oxidizing conditions will result in an aldehyde product.
The Primary alcohols will be oxidized to form the aldehydes and the carboxylic acids. The secondary alcohols will be oxidized to give the ketones. The Tertiary alcohols, in the contrast, cannot be oxidized by without breaking the molecules of the C–C bonds.
The Primary alcohols and the aldehydes will be normally oxidized to the carboxylic acids using the potassium dichromate solution in the presence of the dilute sulfuric acid that is H₂SO₄.
To learn more about oxidation here
https://brainly.com/question/21795438
#SPJ4
the equilibrium constant kc equals 0.0085 for the following reaction at 89oc. ch3oh(g) ⇄ co(g) 2h2(g) what is the value of kp at this temperature?
Answers
The equilibrium constant kc equals 0.0085 for the following response at 89°C. \(CH_{3} OH(g)\) ⇄ \(CO(g) +2H_{2} (g)\) the value of the equilibrium constant (Kp) at 89°C is 0.016 \(atm^2\).
To find the relationship between the equilibrium constant Kc and the equilibrium constant Kp for a response,
we need to use the following equation:
Kp = \(Kc(RT)^{\delta\:n}\)
where R is the gas constant,
T is the temperature in Kelvin, and
\(\delta\:n\) shows the difference in the number of moles of gas between the products and the reactants.
For the given reaction, the balanced equation is as shown below:
\(CH_{3} OH(g)\) ⇄ \(CO(g) +2H_{2} (g)\)
The difference in the number of moles of gas is calculated as:
\(\delta\:n\) = (1 + 2) - 1
\(\delta\:n\) = 2
where we count the coefficients of the gaseous products and deduct the coefficient of the gaseous reactant.
By placing the given values into the equation:
Kp = \(Kc(RT)^{\delta\:n}\)
Kp = \((0.0085) (0.0821\: L\:atm/mol\:K)^2 (362.15 K)\)
Kp = 0.016 \(atm^2\)
For more questions on equilibrium constant
https://brainly.com/question/19340344
#SPJ4
what does LUCA stand for
Answers
pls pls help due in an hour if u get it right i’ll mark u branliest

Answers
The compound is hydrogen peroxide as shown.
How do you know a model of hydrogen peroxide?A molecular model is a representation of a molecule that is used to better understand its properties, behavior, and interactions. Molecular models can be physical, such as models made from plastic or metal, or they can be virtual, such as computer-generated models.
The purpose of a molecular model is to provide a visual and often interactive representation of a molecule's structure, which can aid in the understanding of its chemical and physical properties, as well as its behavior in different environments or under different conditions.
Learn more molecular model:https://brainly.com/question/30545244
#SPJ1
Which of the following is not considered a base unit according to the SI units of measurement

Answers
gram is not the SI unit of measurement. Thus option A is correct.
what is unit of measurement?
The unit of measurement can be defined as the magnitude of quantity that is used as the measurement for the same form, adopted and specified by the law or convention.
We can quantify different types of measurement by multiple measuring unit and this unit is called as the standard quantity of the physical property can be used as the factor for expressing the quantity of that specific.
There are apparent limitations of single unit of measurement like the use of the measurement of the same unit for the distance between two cities and the length of a pencil is impractical.
On the other hand length and weight, and volume are measured in different unit system like CGS system of units, the FPS system of units, the MKS system of units, and the SI system of units etc.
Learn more about SI unit, here:
https://brainly.com/question/12750330
#SPJ2
which 2 particles have opposite sides
Answers
Answer: Protons and electrons
Explanation: Protons are positively charged and electrons are negatively charged.
Answer: protonproton andand electron
Explanation:
ProtonProton isis positivelypositively chargedcharged andand electornelectron isis negativelynegatively chargedcharged withwith massmass numbernumber 11 forfor each
Barium sulfide decomposes into its
elements when heat and electricity are
applied.
Which reaction shows the balanced
equation for the decomposition?
A. 8BaS→ 8Ba + Sg
B. Ba₂S → 2Ba + S
C. BaS2
Ba + S₂
->
D. 2Ba₂S 4Ba + 2S
->
MARINA VINNSAMAN

Answers
A balanced equation obeys the law of conservation of mass. Here the balanced equation for the decomposition of Barium sulfide is 8 BaS → 8Ba + S₈. The correct option is A.
What is balanced equation?A chemical equation in which the amount of reactants and products on both sides of the equation are equal is defined as the balanced equation. The number of atoms of each element of the reactants and products are same on either side of the equation.
Here the balanced equation for the decomposition of Barium sulfide is denoted as:
8BaS→ 8Ba + S₈
The number of 'Ba' and 'S' atoms on both sides of the equation are equal.
Thus the correct option is A.
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ1
A jug contains 2L of milk. Calculate the volume of the milk in m3
Answers
Answer: 0.002 m³
Explanation:
We can use our unit conversions to find the volume in m³.
\(2L*\frac{1m^3}{1000L} =0.002m^3\)
Balanced equation for CaPO4
Answers
Answer:
Ca3(PO4)2 = P2O5 + CaO - Chemical Equation Balancer.
Explanation:
Answer:
Ca3(PO4)2
Explanation:
What always occurs during a nuclear fission reaction?.
Answers
Hey, I’ll give brainliest. Just please help.

Answers
Answer:
C
Explanation:
what is the wavelength range of photons that produce 40-kev electrons in compton scattering?
Answers
The wavelength range of photons that produce 40-keV electrons in Compton scattering is essentially the same as the initial wavelength of the photons.
In Compton scattering, a photon interacts with an electron, transferring some of its energy and momentum to the electron. The change in energy of the photon is related to the scattering angle through the Compton wavelength shift equation:
Δλ = λ' - λ = h / (mec) * (1 - cos(θ))
where Δλ is the change in wavelength, λ' is the scattered wavelength, λ is the initial wavelength, h is the Planck constant, me is the electron mass, c is the speed of light, and θ is the scattering angle. Given that the energy of the electron is 40 keV, use the equation for the energy of a photon to determine the initial photon energy:
E = hc / λ
40,000 eV = (hc / λ') - (hc / λ)
Simplifying the equation:
hc / λ' = (hc / λ) + 40,000 eV
λ' = (λ * λ') / (λ - λ')
Substituting λ' = λ - Δλ, we get:
λ - Δλ = (λ * (λ - Δλ)) / λ
Simplifying further:
λ - Δλ = λ - Δλ
This equation indicates that the change in wavelength is negligible compared to the initial wavelength.
Learn more about Compton scattering here:
https://brainly.com/question/29306626
#SPJ11
what is not a common automotive fuel
Answers
Answer:
Hydrogen
Explanation:
A stream containing 30% ethanol (molecular weight 46) and the rest is water (molecular weight 18). The mass flow rate of the stream is 100 kg/h. The molar flow rate of water is:
Answers
Based on the mass flow rate of the mixture, the molar flow rate of water is 3.89 mol/h
What is the molar flow rate of a fluid?The molar flow rate of a material refers to the number of moles of a material that passes through a given point of reference within a unit time interval.
The molar flow rate = moles / time
From the data provided:
The mass flow rate of the mixture is 100 kg/h
Percent mass of water = 70%
Hence mass flow rate of water = 70 g/h
molar mass of water = 18 g/mol
Molar flow rate of water = 70 g/h ÷ 18g/mol
Molar flow rate of water = 3.89 mol/h
Learn more about molar flow rate at: https://brainly.com/question/26061120
#SPJ1
What are the criteria of high-quality scientific research?
Provide at least three examples and explain them in detail.
Answers
High-quality scientific research is characterized by several key criteria. Three examples of such criteria include: rigorous experimental design and methodology, reliable data analysis and interpretation, and clear and transparent reporting of results.
These criteria ensure that research is conducted in a systematic and reliable manner, leading to trustworthy and valid findings.
Rigorous Experimental Design and Methodology: High-quality scientific research requires a well-designed experimental approach. This involves careful planning, proper control groups, randomization, and replication. A rigorous methodology ensures that experiments are conducted under controlled conditions, minimizing bias and confounding variables, and allowing for accurate and reliable data collection.
Reliable Data Analysis and Interpretation: After data collection, high-quality research involves thorough and appropriate analysis of the data. This includes using appropriate statistical methods to evaluate the significance of the results and drawing valid conclusions. Proper data analysis helps researchers identify patterns, trends, and relationships, supporting or refuting their hypotheses in an objective and reliable manner.
Clear and Transparent Reporting of Results: High-quality research demands transparent reporting of the methods, procedures, and findings. This includes providing detailed descriptions of the experimental setup, data collection processes, and statistical analyses used. Clear reporting allows other researchers to replicate the study and verify its results. Additionally, complete reporting ensures that readers can understand the research methodology and draw their own conclusions based on the evidence presented.
By adhering to these criteria, high-quality scientific research maintains integrity, credibility, and reproducibility. It fosters trust among the scientific community and facilitates the advancement of knowledge by building upon reliable foundations.
To learn more about research click here:
brainly.com/question/31251355
#SPJ11
a 355-ml can of soda pop contains 41 g of sucrose (c12h22o11). what is the molarity of the solution with respect to sucrose?
Answers
A 355-ml can of soda pop contains 41 g of sucrose the molarity of the solution with respect to sucrose is 0.337 M
Given that 41g of sucrose
Number of moles = amount in g/ molar mass
Number of moles= 41 g / 342.2965 g/mol
Number of moles= 0.12 moles Sucrose
Volume = 355 ml or 0.355 L
Thus,
Molarity = 0.12 moles / 0.355 L
Molarity= 0.337 M
The term "molarity of the solution" refers to how many moles of solute there are in a liter of solution. M served as its symbol. The ratio of moles of solutes per liter of solution is known as the molarity of any solution. The following formula captures it.
Molarity (M) = moles of solute/liter of solution
Learn more about molarity here:
https://brainly.com/question/2817451
#SPJ4
why do you think the pressure changed as it did when the temperature was increased?
Answers
Answer:
La Convención Marco de las Naciones Unidas sobre el Cambio Climático (CMNUCC) en su Artículo 1, lo define como ‘un cambio de clima atribuido directa o indirectamente a la actividad humana que altera la composición de la atmósfera mundial y que se suma a la variabilidad natural del clima observada durante períodos de tiempo comparables’. La CMNUCC distingue entre ‘cambio climático’ atribuido a actividades humanas que alteran la composición atmosférica y ‘variabilidad climática’ atribuida a causas naturales.
Los científicos han encontrado evidencias de que el clima en el planeta está cambiando a un ritmo más acelerado de lo esperado y que nuestras actividades ligadas a la producción, extracción, asentamiento y consumo, son la principal causa de este aceleramiento en el cambio.
El mayor problema de un cambio acelerado en el clima es que nuestras sociedades no están preparadas para asumir los cambios que esto nos pueda traer: derretimiento de las masas glaciares y nevados que abastecen acueductos, cambios en los ciclos de floración y fructificación de las plantas de cultivo, ascensos en el nivel de los mares donde hay mucha población viviendo, mayor ocurrencia y fuerza en lluvias, sequías, huracanes, heladas y granizadas en áreas urbanas y rurales, entre otros fenómenos que sin duda reducen nuestra calidad de vida.
Explanation:
espero te áyude

a carbonate ion, co32-, can participate in an acid-base reaction. how should the carbonate ion be classified?
Answers
The carbonate ion is classified as amphiprotic, meaning it can act as both an acid and a base in a chemical reaction.
The carbonate ion (CO₃²⁻) is a polyatomic ion composed of one carbon atom and three oxygen atoms. It has a negative charge of 2⁻, which is balanced by one or more positive ions in ionic compounds.
The carbonate ion is a versatile species that participates in various chemical reactions. For example, it can react with acids to form bicarbonate (HCO₃⁻) or carbonic acid (H₂CO₃). It is also a key component of many minerals, such as limestone, calcite, and dolomite.
The carbonate ion, CO₃²⁻, can participate in an acid-base reaction and can act as a base, accepting a proton from an acid to form bicarbonate (HCO₃⁻) or as an acid, donating a proton to a base to form carbonic acid (H₂CO₃).
To know more about carbonate ion here
https://brainly.com/question/13878773
#SPJ4
does a 700 nm photon have more or less energy than a 400 nm photon? more less correct: your answer is correct. by what factor more (or less)? enter your answer as e700 nm e400 nm . e700 nm e400 nm
Answers
It is the 400 nm photon that has the greater energy by a factor of 1.77.
What is the wavelength?We know that the wavelength has to do with the horizontal distance that is covered by a wave. We know that the longer the distance that is covered then the weaker the wave. In other words, the wavelength of the wave is inversely proportional to the to the energy of the wave. The greater the wavelength, the lesser the energy.
Let us now find the energy of each of the wavelengths;
E = hc/λ
E = energy of the light
h = Plank's constant
c = speed of light
λ = wavelength
For the 700 nm photon;
E = 6.6 * 10^-34 * 3 * 10^8/ 700 * 10^-9
E = 2.8 * 10^-19 J
For the 400 nm photon;
E = 6.6 * 10^-34 * 3 * 10^8/ 400 * 10^-9
E= 4.95 * 10^-19 J
Learn more about energy of a photon:https://brainly.com/question/11016364
#SPJ1
A giraffe is found in the African grasslands. Is this an example of a habitat or a niche
Answers
Answer:
Niche i think
Explanation:
90% of people marry there 7th grade love. since u have read this, u will be told good news tonight. if u don't pass this on nine comments your worst week starts now this isn't fake. apparently if u copy and paste this on ten comments in the next ten minutes you will have the best day of your life tomorrow. you will either get kissed or asked out in the next 53 minutes someone will say i love you
In order to convert to Kelvin you add blank to the Celsius measurement
Answers
In order to convert to Kelvin we add 273 to the Celsius measurement as we know that 0 degree Celsius is equal to 273Kelvin.
Celsius (°C) and Kelvin (K) are both units of temperature measurement. The difference between the two is that Kelvin is an absolute scale, and Celsius is a relative scale. Kelvin scale is an absolute temperature scale, which means that it starts at absolute zero. Absolute zero is defined as 0 Kelvin, or -273.15 Celsius. Therefore, to convert from Celsius to Kelvin, you add 273.15 to the Celsius measurement.
For example, if the temperature is 100 degree Celsius, then to convert it to Kelvin, you would add 273.15 to 100, which would give a result of 373.15 Kelvin.
To learn more about temperature click here https://brainly.com/question/29072206
#SPJ4
Which statement is incorrect about the setup of voltaic cell? a.A voltaic cell is an electrochemical cell that uses spontaneous redox reactions to generate electricity. It consists of two separate half-cells. A salt bridge also connects to the half cells. b.Salt bridge is a tube usually filled with an electrolyte solution such as KNO3(s) or KCI(s) c.The salt bridge allows a flow of ions that neutralizes the charge build up in the solution. d.In Voltaic cells, oxidation occurs at cathode and reduction occurs at anode.
Answers
The incorrect statement about the setup of a voltaic cell is (d) "In voltaic cells, oxidation occurs at the cathode and reduction occurs at the anode."
In a voltaic cell, oxidation actually occurs at the anode and reduction occurs at the cathode. This is because electrons flow from the anode (where oxidation takes place) to the cathode (where reduction takes place). The anode is the electrode where oxidation reactions take place and electrons are released, while the cathode is the electrode where reduction reactions occur and electrons are gained. To explain further, in a voltaic cell, the anode is the electrode where the oxidation half-reaction occurs. Oxidation involves the loss of electrons and the anode serves as the source of electrons. These electrons then flow through an external circuit to the cathode. At the cathode, reduction takes place, which involves the gain of electrons. The cathode acts as the site where reduction half-reactions occur, consuming the electrons that flow from the anode. Therefore, the correct statement should be: "In voltaic cells, oxidation occurs at the anode and reduction occurs at the cathode."
learn more about voltaic cell here: brainly.com/question/31729529
#SPJ11
When you watch a sunset, is the Sun really moving across the sky? What's happening?
(Science)