Answers
P4(s) + 6Cl2(g) → 4PCl3(l)
n P4 = m P4 / Mr P4
n P4 = 18.74 / 123.88
n P4 = 0.151 mol
n Cl2 = m Cl2 / Mr Cl2
n Cl2 = 9.6 / 70.9
n Cl2 = 0.135 mol
Cl2 act as limiting reactant
n PCl3 = (coef. PCl3)/(coef. Cl2) • n Cl2
n PCl3 = (4/6) • 0.135
n PCl3 = 0.09 mol
m PCl3 = n PCl3 • Mr PCl3
m PCl3 = 0.09 • 137.33
m PCl3 = 12.36 gr
Related Questions
Predict and explain the structure of the major and minor products when hydrogen bromide is added to 2-methylbut-2- ene, (Ch3)2CCHCH3
Pls help with homework!!!!
Answers
When hydrogen bromide (HBr) is added to 2-methylbut-2-ene ((CH3)2CCHCH3), an electrophilic addition reaction takes place, where the π bond of the alkene is broken, and the hydrogen and bromine atoms are added to the resulting carbocation.
The reaction proceeds through a Markovnikov addition, where the hydrogen atom attaches to the carbon atom with the greater number of hydrogen atoms.
In this case, the initial addition of HBr to 2-methylbut-2-ene leads to the formation of a primary carbocation, as the positively charged carbon atom only has one alkyl group attached to it. The primary carbocation is relatively unstable, and it can undergo a rearrangement to form a more stable secondary carbocation.
The major product that is typically obtained is the 2-bromo-2-methylbutane. The hydrogen atom from HBr adds to the carbon with three hydrogen atoms (the more substituted carbon), resulting in the formation of a secondary carbocation.
On the other hand, a minor product is also formed, which is 3-bromo-2-methylbutane. This product arises from the addition of HBr to the primary carbocation, which is less stable. Although the primary carbocation is less favored, it can still be formed and lead to the formation of the minor product.
In summary, the addition of HBr to 2-methylbut-2-ene yields two products: the major product is 2-bromo-2-methylbutane, resulting from the addition of HBr to the more stable secondary carbocation, and the minor product is 3-bromo-2-methylbutane, originating from the less stable primary carbocation.
For more such questions on electrophilic addition visit:
https://brainly.com/question/9643304
#SPJ8
The escape of molecules from the surface area of a liquid is known as
Answers
Answer:
Evaporation is a type of vaporization of a liquid that only occurs on the liquid's surface. Usually, the molecules in a glass of water do not have enough heat energy to escape from the liquid. With sufficient heat, however, the liquid would quickly turn into vapor.
Explanation:
Evaporation is a type of vaporization of a liquid that only occurs on the liquid's surface. Usually, the molecules in a glass of water do not have enough heat energy to escape from the liquid. With sufficient heat, however, the liquid would quickly turn into vapor.
The Ka of a monoprotic weak acid is 0.00469. What is the percent ionization of a 0.141 M solution of this acid?
Use quadratic equation.
Answers
The percent ionization of an acidic solution can be calculated from the H+ concentration. the percent ionization of the monoprotic acid of 0.141 M is 18.23 %.
What is percent ionization?Percent ionization of an acidic solution is the percent of H+ ions in the solution. Thus, mathematically, it is the ratio of H+ ion concentration to the concentration of solution multiplied by 100.
Let HA be the monoprotic acid when it ionizes, forming equal concentration of H+ and A- let it be x. Thus ionization constant can be written as follows:
Ka = [x]² /[HA]
0.00469 =[x]²/[0.141 M]
[X] = 0.025. = [H+]
Percentage ionization = (0.025 M / 0.141 M)× 100
= 18.23 %
Therefore percentage ionization of the acid is 18.23%.
To find more about percentage ionization, refer the link below:
https://brainly.com/question/11064341
#SPJ1
What is the purpose of a lab report?
a. to collect your experimental data
b. to design your experiment
c. to do your preliminary literature search
d. to provide information on your experiment in a clear, concise,
and consistent format
Answers
The purpose of a lab report is to provide information about an experiment in a clear, concise, and consistent format. Therefore, option (D) is correct.
What is a lab report?The lab report can be described as a paper that documents the outcome of an experiment. People create this form of paper to demonstrate the student's understanding of the experimental procedure and the result's relevance.
The primary goal of this is not the documentation of the process but the illustration of the theory behind the experimental procedure and its ability to describe the behavior of variables. lab reports are described as comprehensive documentation of the entire experimentation process including theoretical analysis.
Therefore, lab reports are prepared to provide information about an experiment in a clear, concise, and consistent pattern.
Learn more about Lab reports, here:
https://brainly.com/question/24553469
#SPJ1
element is responsible for the color of light produced.
13
In a flame test, the
a Non-metal
b. Metal
c. Halogen
d. Noble Gas
1. Use the table to
Answers
Answer: d
Explanation:because it makes sence i think
In a protein molecule, the number of amino acid molecules may be as few as
5
5,000
500
50
Answers
In a protein molecule, the number of amino acid molecules may be as few as d. 50.
Proteins are large macromolecules composed of chains of amino acids. These amino acids are linked together through peptide bonds to form the primary structure of a protein. The number of amino acids present in a protein molecule can vary greatly and depends on the specific protein.
Proteins can range in size from small peptides composed of just a few amino acids to large complex proteins containing thousands of amino acids. The minimum number of amino acids required for a protein to be considered functional is typically around 50. This is because proteins need a certain length and structural complexity to carry out their specific functions.
Proteins play vital roles in living organisms and are involved in various biological processes such as enzyme catalysis, cell signaling, structural support, and immune response. The specific number and sequence of amino acids in a protein molecule determine its unique structure and function.
Therefore, while some proteins may consist of as few as 50 amino acids, larger and more complex proteins can contain thousands of amino acids, enabling them to perform intricate and diverse functions within living systems. Therefore, Option D is correct.
The question was incomplete. find the full content below:
In a protein molecule, the number of amino acid molecules may be as few as
a. 5
b. 5,000
c. 500
d. 50
Know more about amino acid here:
https://brainly.com/question/14351754
#SPJ8
Given 2AL + 6HCL → 2ALCL3 + 3H2, how many grams of aluminum do I need to produce 11 L of hydrogen gas at STP?
Answers
Mass of Aluminum= 8.829 g
Further explanationGiven
Reaction
2Al + 6HCl → 2AlCl₃ + 3H₂
Required
mass of Aluminum
Solution
At STP, 1 mol gas = 22.4 L
For 11 L of Hydrogen :
= 11 : 22.4
= 0.491
From equation, mol ratio Al : H₂ = 2 : 3, so mol Al :
= 2/3 x mol H₂
= 2/3 x 0.491
= 0.327
Mass Aluminum(Ar=27 g/mol) :
= 0.327 mol x 27 g/mol
= 8.829 g
How does adding and taking away electrons change the charge of an atom?
Answers
Answer: If a neutral atom gains electrons, then it will become negatively charged
Explanation: If a neutral atom gains electrons, then it will become negatively charged. If a neutral atom loses electrons, then it become positively charged.
Whith of the following numbers is biger than 9.20 x 10-7?
Answers
Answer:
(9.20×10)-7
92-7
=85
Explanation:
Multiplication comes first in BODMAS
Hope it helps
ANSWER ALL - OVERDUE
Which of the following statements about salinity is true? Lesson 2.02
Question 1 options:
Ocean water in areas with high humidity has a higher salinity.
Ocean water near rivers has a lower salinity.
Ocean water in regions with high levels of precipitation has higher salinity.
Ocean water near areas with low evaporation has higher salinity.
Question 2 (1 point)
Saved
How are latitude and temperature related? (Lesson 2.03)
Question 2 options:
Lower latitudes will have warmer water because it is closer to the equator
Higher latitudes will have warmer water because it is closer to the poles.
Higher latitudes will have warmer water because it is closer to the equator.
Question 3 (1 point)
Saved
How does salinity vary with freezing and melting? (Lesson 2.02)
Question 3 options:
Both freezing and melting increase salinity.
Freezing increases salinity, while melting decreases salinity.
Both freezing and melting decrease salinity.
Freezing decreases salinity, while melting increases salinity.
Question 4 (1 point)
How does salinity vary with evaporation? (Lesson 2.02)
Question 4 options:
When water evaporates, it leaves salt behind, increasing its salinity.
When water evaporates, it takes salt with it, decreasing its salinity.
When water evaporates, it takes salt with it, increasing its salinity.
When water evaporates, it leaves salt behind, decreasing its salinity.
Question 5 (1 point)
Saved
What is density? (Lesson 2.02)
Question 5 options:
the amount of salt in water
the weight of water
a measure of salinity
the amount per unit volume of a particular material
Question 6 (1 point)
Saved
As the amount of salt in water increases, the___________ of the water increases. (Lesson 2.02)
Question 6 options:
temperature
humidity
size
density
Question 7 (1 point)
Deep, cold water is the __________. Warm, shallow water is the ___________. (Lesson 2.03)
Question 7 options:
densest; densest
least dense; densest
densest; least dense
least dense; least dense
Question 8 (1 point)
Which layer of the ocean is the least dense? (Lesson 2.03)
Question 8 options:
closest to the ocean floor
closest to the surface
middle layer
they are all the same
Question 9 (1 point)
The _______________makes things (like planes or currents of air) traveling long distances around Earth appear to move at a curve as opposed to a straight line. (Lesson 2.05)
Question 9 options:
Aurora
Coriolis Effect
Salinity effect
Temperature
Question 10 (1 point)
The main cause of wind is differences in temperature in different areas. (Lesson 2.04)
Question 10 options:
True
False
Answers
Statements on salinity;
Ocean water near rivers has a lower salinity, B.Lower latitudes will have warmer water because it is closer to the equator, A.Freezing increases salinity, while melting decreases salinity, A.When water evaporates, it leaves salt behind, increasing its salinity, B.The amount per unit volume of a particular material, D.Density, D.Densest; least dense, C.Closest to the surface, B.Coriolis Effect, B.True.What is Salinity?Salinity refers to the measure of the concentration of dissolved salts, such as sodium and chloride ions, in a body of water. It is usually expressed in parts per thousand (ppt) or practical salinity units (PSU).
Salinity can vary in different bodies of water due to factors such as evaporation, precipitation, freshwater input from rivers, and ocean currents.
Learn more on salinity here: https://brainly.com/question/20283396
#SPJ1
How did the work of Dmitri Mendeleev differ from that of John Newlands in the development of the periodic table?
Answers
Answer: Mendeleev predicted elements that would later be discovered.
Bohr's model was correct in assigning energy levels to electrons.
Answers
Answer:
yes that is true........
5.
The reaction 2AB 2A + B is first order with respect to AB.
The half-life of the reaction is 2 minutes.
0.100 mol of AB is dissolved in a solvent to form 100 cm of a reaction mixture.
What is the concentration of AB, in mol dm, after 6 minutes?
A. 0.0125
B. 0.0250
CU 0.125
D. 0.250
[1]
Your answer
Answers
The concentration of AB, in mol/dm³, after 6 minutes : 0.125
Further explanationFor first-order reaction :
\(\tt [A]=[A]oe^{-kt}\rightarrow t1/2=\dfrac{ln~2}{k}\)
The half-life of the reaction is 2 minutes⇒t1/2=2 minutes
The concentration of AB, in mol dm, after 6 minutes ⇒ t=6 minutes
The rate constant (k) :
\(\tt k=\dfrac{ln~2}{t1/2}=\dfrac{0.693}{2}=0.3465\)
The concentration after 6 minutes :
\(\tt [A]o=0.1~mol/100~cm^3=1~~mol/dm^3\\\\(A]=1\times e^{-0.3465\times 6}\\\\(A]=0.125\)
in a triple beam balance how do you know when you have to move a weight back to the previous notches or grooves
Answers
Answer:
sdipgjaeri0ae
Explanation:
trachea
Meaning of trachea
Answers
Answer:
The trachea, also called the windpipe, is a cartilaginous tube that connects the larynx to the bronchi of the lungs, allowing the passage of air, and so is present in almost all air-breathing animals with lungs. The trachea extends from the larynx and branches into the two primary bronchi. At the top of the trachea the cricoid cartilage attaches it to the larynx. The trachea is formed by a number of horseshoe-shaped rings, joined together vertically by ligaments over their substance and by the trachealis muscle at their ends. The epiglottis closes the opening to the larynx during swallowing.
Explanation:
You asked I answered.
Hope this helped! :)
P.S. If you still don't get where the trachea is, look at the graph below. :)

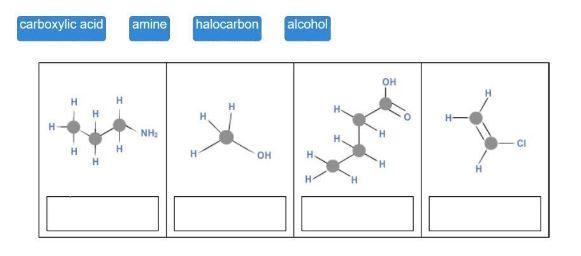
Match each hydrocarbon class to its structure.
4
carboxylic acid
H
H
HT
H
H
H
amine
NH₂
halocarbon
H
OH
alcohol
H.
H
H.
'H
OH
'H
H-
-CI
Answers
The tile's suggested answers include amine, alcohol, carboxyl group, and halocarbon.
Gasoline is it a hydrocarbon?Hydrocarbons are organic substances comprised of hydrogen and carbon, and include petroleum, methane gas, and coal. Alkanes are both a highly combustible chemical and the main source of fuel in the planet. Its uses include diesel, jet fuel, propane, petrol, and petroleum, to name a few.
What makes it a hydrocarbon?The most fundamental category of organic compounds is referred to as a hydrocarbon. As their name implies, they are exclusively made up of the elements hydrogen and carbon. Atoms surround one or more core carbon atoms in hydrocarbon molecules, which are branching or chain-like in shape.
To know more about Hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ1
The complete question is-
Drag each tile to the correct image. Match each hydrocarbon class to its structure. carboxylic acid amine halocarbon alcohol.
Help me pls ima faill plss

Answers
Answer:
C3H8 + 5O2 = 3Co2 + 4 H2o
Explanation:
we can balance this equation by algebric method ( ABC method)
List the 2 pKa's for H2SO4
Answers
In the reaction 2H2(g)+02(g)=2H2O(g) what’s the elements in terms of numbers of molecules,moles,and volume of gases at STP
Answers
In this question, we have the following reaction:
2 H2 + O2 -> 2 H2O
At STP (standard temperature and pressure), 1 mol of gas is equal to 22.4 Liters of volume, and also equal to 6.022*10^23 molecules or particles, and this is the Avogadro's constant number. For our question, we have 2 moles of H2, 1 mol of O2 and 2 moles of H2O, according to this, we will have:
1.204*10^24 molecules of H2
2 moles of H2
44.8 L of H2
6.022*23 molecules of O2
1 mol of O2
22.4 L of O2
1.204*10^24 molecules of H2O
2 moles of H2O
44.8 L of H2O
please help illl mark brainlst (no links)
seafloor spreading allows the new formation of rock by:
A ) New rocks floating to the surface of the crust.
B ) The oceanic plates hit each other causing new rocks to fall off.
C ) Magma cooling on the crust.
D ) Seafloor spreading does not create new rocks.
Answers
Answer:
A ) New rocks floating to the surface of the crust.
TRUST
The rotational spectrum of 79BrºF shows a series of equidistant lines spaced 0-714 33 cm - apart. Calculate the rotational constant B, and hence the moment of inertia and bond length of the molecule. Determine the wavenumber of the J = 9+= 10 transition, and find which transition gives rise to the most intense spectral line at room temperature (say 300 K).
and calculate the number of revolutions per second which the Brf molecule undergoes when in (a) the J = 0 state, (b) the J = 1 state, and (c) the J = 10 state. Hint: Use E = {lwin conjunction with Eqs (2.10) and (2.13), but remember that here w is in radians per second.[its Q season 2 from fundamentals of molcular spectruscopy . banwell.c.n]
Answers
In the J = 0 state, the BrF molecule does not undergo any revolutions per second. In the J = 1 state, it undergoes approximately 0.498 revolutions per second, and in the J = 10 state, it undergoes approximately 15.71 revolutions per second.
To calculate the rotational constant B, we can use the formula:
B = 1 / (2 * π * Δν)
Where:
B = rotational constant
Δν = spacing between consecutive lines in the rotational spectrum
Given that the spacing between consecutive lines is 0.71433 cm^(-1), we can substitute this value into the formula:
B = 1 / (2 * π * 0.71433 cm^(-1))
B ≈ 0.079 cm^(-1)
The moment of inertia (I) of the molecule can be calculated using the formula:
I = h / (8 * π^2 * B)
Where:
h = Planck's constant
Given that the value of Planck's constant (h) is approximately 6.626 x 10^(-34) J·s, we can substitute the values into the formula:
I = (6.626 x 10^(-34) J·s) / (8 * π^2 * 0.079 cm^(-1))
I ≈ 2.11 x 10^(-46) kg·m^2
The bond length (r) of the molecule can be determined using the formula:
r = sqrt((h / (4 * π^2 * μ * B)) - r_e^2)
Where:
μ = reduced mass of the molecule
r_e = equilibrium bond length
To calculate the wavenumber (ν) of the J = 9+ to J = 10 transition, we can use the formula:
ν = 2 * B * (J + 1)
Substituting J = 9 into the formula, we get:
ν = 2 * 0.079 cm^(-1) * (9 + 1)
ν ≈ 1.58 cm^(-1)
To determine the most intense spectral line at room temperature (300 K), we can use the Boltzmann distribution law. The intensity (I) of a spectral line is proportional to the population of the corresponding rotational level:
I ∝ exp(-E / (k * T))
Where:
E = energy difference between the levels
k = Boltzmann constant
T = temperature in Kelvin
At room temperature (300 K), the population distribution decreases rapidly with increasing energy difference. Therefore, the transition with the lowest energy difference will have the most intense spectral line. In this case, the transition from J = 0 to J = 1 will have the most intense spectral line.
To calculate the number of revolutions per second, we can use the formula:
ω = 2 * π * B * J
Where:
ω = angular frequency (in radians per second)
J = rotational quantum number
For J = 0:
ω = 2 * π * 0.079 cm^(-1) * 0 = 0 rad/s
For J = 1:
ω = 2 * π * 0.079 cm^(-1) * 1 ≈ 0.498 rad/s
For J = 10:
ω = 2 * π * 0.079 cm^(-1) * 10 ≈ 15.71 rad/s
For more such questiosn on BrF molecule visit;
https://brainly.com/question/30624940
#SPJ8
Consider the following reaction: MgF(2) + 2 Li —> Mg + 2 LiF
How many grams of My could you make from 20.0 g of MgF(2)
These are the molar masses for each :
MgF(2) = 62.3g/mol
Li = 6.9g/mol
Mg = 24.3g/mol
LiF = 25.9g/mol
Answers
Answer:
Explanation:
62.3018064. We assume you are converting between grams MgF2 and mole.
Methylhydrazine, CH6N2 is commonly used as a liquid rocket fuel. The heat of combustion of methylhydrazine is -1.30x10^3 kJ/mol. How much heat is released when 100.0g of methylhydrazine is burned?
Answers
The heat released by 100g of the compound is 2.8 x10^3 kJ .
What is combustion?We know that the process of combustion is the process that enables a substance to be burnt in air. Heat and light can be produced in the process of the combustion.
Number of moles of the Methylhydrazine = mass/ molar mas = 100.0g/46 g/mol = 2.17 moles
If from the balanced reaction equation;
1 moles of Methylhydrazine produces 1.30x10^3 kJ
Then 2.17 moles of Methylhydrazine produces 2.17 moles * 1.30x10^3 kJ/1 mole
= 2.8 x10^3 kJ of heat.
Learn more about heat of combustion:https://brainly.com/question/14317568
#SPJ1
which of the following is NOT a chemical property?
Conductivity
Malleability
Reactivity
Freezing
Answers
Answer:
Malleability
Explanation:
Answer:
Malleability is not chemical property
Some SO2 and O2 are mixed together in a flask at 1100 K in such a way ,that at the instant of mixing, their partial pressures are, respectively, 1.00 atm and 0.500 atm. When the system comes to equilibrium at 1100 K, the total pressure in the flask is found to be 1.35 atm. Given: 2SO2(g) + O2(g) ⇌ 2SO3(g); ΔrH = − 198.2 kJ. mol-1 1.1 Calculate Kp at 1100 K
Answers
Answer:
The answer is "\(\bold{0.525\ \ atm^{-1}}\)"
Explanation:
Given equation:
\(2SO_2(g) + O_2(g) \leftrightharpoons 2SO_3(g)\)
Given value:
\(\Delta rH =-198.2 \ \ \frac{KJ}{mol}\)
\(Kp=1100 \ K\)
\(\Delta x = 2-(2+1)\\\\\)
\(= 2-(2+1)\\\\= 2-(3)\\\\= -1\)
\(\left\begin{array}{cccc}I\ (atm)&1&0.5&0\\C\ (atm)&2x&-x&2x\\E\ (atm) &1-2x&0.5-x&2x\end{array}\right\)
calculating the total pressure on equilibrium= \((1-2x)+(0.5-x)+2x \ atm\\\\\)
\(= 1-2x+0.5-x+2x\\\\= 1+0.5-x\\\\=1.5-x\\\)
\(\therefore \\\\\to 1.50-x= 1.35 \\\\\to 1.50-1.35= x\\\\\to 0.15= x\\\\ \to x= 0.15\\\\\)
calculating the pressure in \(So_2\):
\(= (1-2 \times 0.15)\)
\(= 1-0.30 \\\\ =0.70 \ atm\)
calculating the pressure in \(O_2\):
\(= (0.5- 0.15)\\\\= 0.35 \ atm \\\)
calculating the pressure in \(So_3\):
\(= (2 \times 0.15)\\\\= (.30) \ atm \\\\\)
Calculating the Kp at 1100 K:
\(= \frac{(Pressure(So_3))^2}{(Pressure(So_2))^2 \times Pressure(O_2)}\\\\= \frac{0.30^2}{0.70^2 \times 0.35}\\\\= \frac{0.30 \times 0.30 }{0.70\times 0.70 \times 0.35}\\\\= \frac{0.09 }{0.49\times 0.35} \\\\= \frac{0.09 }{0.1715} \\\\= 0.5247 \ \ or \ \ 0.525 \ \ atm^{-1} \\\\\)
The water vapor that condenses low to the ground and becomes visible is known as ___________.
Answers
I’m sorry if I’m wrong but I’m pretty sure that’s the answer:)
The data in the table below represents the pressure of the gas as the temperature changes. Plot a graph of this data, using the blank graph provided below. Draw a trend line and calculate its slope. How are the variables related? What will the pressure of the gas be at 0°C?
Answers
Answer: A
Explanation:
Which of the following is a primary source?
A. A newspaper article
B. A news report on television
O C. A scientific journal article
O D. An encyclopedia entry
Answers
Answer:
I think its C a scientific journal article
Explanation:
Any kind of journal is considered a primary source because journals contain info that the original author wrote. Encyclopedias are considered teritary sources, but im not sure if that counts ...so id go with journals.
A scientific journal article is a primary source. Therefore, the correct option is option C among all the given options.
What is primary source?A primary source, also known as an original source, in the academic study of history is any item, document, journal, manuscript, biography, recording, or other source of data that was produced during the period being examined.
It acts as an authentic resource for knowledge on the subject. Although several domains have slightly distinct definitions, library science as well as other areas of academia might utilize comparable terms. A primary source in journalism might be a person who has firsthand knowledge of an event or a written account of it. A scientific journal article is a primary source.
Therefore, the correct option is option C.
To know more about primary source, here:
https://brainly.com/question/29771183
#SPJ7
What is the nature of soil if pH value of soil is 6?
Answers
Answer:
strongly acidic
Explanation:
Soils can be classified according to their pH value: 6.5 to 7.5—neutral. over 7.5—alkaline. less than 6.5—acidic, and soils with pH less than 5.5 are considered strongly acidic.
If your typing this I would recommend that you don't copy this word for word because of plagerism I dont want to be the source for that. You may alternate it and put it in your own words tho :)
1. Which macromolecules would you need if... you need a quick boost of
energy?
Answers
Answer:
\(\boxed {\tt Carbohydrate \ or \ monosaccharides}\)
Explanation:
There are 4 main types of macromolecules: lipids, proteins, carbohydrates, and nucleic acids.
If you are looking for some quick energy, the best choice is carbohydrates. Carbohydrates are the easiest for the body to break down and turn into ATP/energy.
There are also different types of carbohydrates. If you want a more specific answer, monosaccharides is the best answer. They are the simplest sugars. Also, glucose is a monosaccharide, and it shows up directly in the cellular respiration formula. If glucose is consumed, the body doesn't have to break it down before starting respiration.