1
How many individual oxygen atoms are contained in a sample of P₂O5 that also
contains 0.620 moles of P? (Input your answer with scientific notation using "e
Answers
9.331 x \(10^{23}\) many individual oxygen atoms are contained in a sample of P₂O5 that also contains 0.620 moles of P.
What are moles?A mole is defined as 6.02214076 × \(10^{23}\)of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance.
\(P_2O_5\) → \(2P^+{^5} + 5O{^-^2}\)
Given:
0.620 moles of P
Let us assume a mole of oxygen atoms.
2÷0.620 = 5÷a Cross Multiply
2a = 0.620 x 5 Divide both sides by 2
a= 0.310 x 5 Combine
a = 1.55 moles of oxygen.
Atoms are there in 1.55 moles of oxygen:
1 mole of anything contains 6.02 x \(10^{23}\) items of that mole
1.55 moles of oxygen contain x atoms of oxygen
1÷1.55 = 6.02 x \(10^{23}\) ÷x Cross multiply
1 x a = 1.55 x 6.02 x \(10^{23}\) Combine
a = 9.331 x \(10^{23}\)
Hence, 9.331 x \(10^{23}\) many individual oxygen atoms are contained in a sample of P₂O5 that also contains 0.620 moles of P.
Learn more about moles here:
https://brainly.com/question/8455949
#SPJ1
Related Questions
A sample of gas at an initial volume of 4.2 L an initial pressure of 1.12 atm, and an initial temperature of 186 K simultaneously changes its temperature to 355 K and its pressure to 0.82 atm What is the final volume of the gas? [3 SF) (10.91)
Answers
(P1 * V1) / T1 = (P2 * V2) / T2
Where P1, V1, and T1 represent the initial pressure, volume, and temperature, and P2, V2, and T2 represent the final pressure, volume, and temperature.
Substituting the given values into the equation, we have:
(1.12 atm * 4.2 L) / 186 K = (0.82 atm * V2) / 355 K
Simplifying the equation:
4.704 atm*L/K = (0.82 atm * V2) / 355 K
Cross-multiplying:
4.704 atmLK = 0.82 atm * V2
Dividing both sides by 0.82 atm:
5.739 atmLK / atm = V2
Simplifying:
V2 = 5.739 L
Therefore, the final volume of the gas is 5.739 L (rounded to three significant figures).
Draw the lewis structure for each of the following - letter ba) NF3b) ClO3-c) HOBrd) SO3-2
Answers
Answer:
Explanation:
The question requires us to draw the Lewis structure for ClO3-.
In order to draw the Lewis structure of a molecule or ion, we need to consider the number of valence electrons in each atom of the structure:
O presents electron configuration: 1s2 2s2 2p4, thus it contains 6 valence electrons.
Cl presents electron configuration: 1s2 2s2 2p6 3s2 3p5, thus it contains 7 valence electrons.
Now, we can start drawing the Lewis structure for ClO3-.
1) First, we need to choose a central atom. Let's consider Cl as there is only one atom of it:
2) Next, we can "add" the electrons between the outer atoms and the central atom, representing bonds:
3) Now, let's complete the electrons on the atoms, starting with the outer atoms and then filling the central atom. Note that the total electrons is 3*6 + 1*7 = 25 electrons.



Explain how the carbon and oxygen atoms from co2 molecules in the air become part of the cellulose molecules in plant cell walls.
Answers
Your carbon atom enters the leaf as atmospheric CO2, or carbon dioxide.
6CO2 + 6H2O = C6H12O6 + 6O2.
Briefing:
Chlorophyll in the leaf uses the sun's energy to change CO2 and H2O into the sugar glucose, C6H12O6. This molecule contains your carbon atom, which is now one of the carbons in the glucose.
The glucose bearing your carbon atom is then transported to the carrot's root by the phloem tissue of the carrot plant. The glucose molecule with your carbon atom is connected by enzymes in the carrot root to a chain of other glucose molecules to create cellulose, or plant starch, and there you have it. Through photosynthesis, your carbon atom transitioned from being a component of an atmospheric gas to a starch.
To know more about enzymes, visit:
https://brainly.com/question/14953274
#SPJ4
what is a covalent and ionic bond
Answers
Answer:
Covalent bonding involves the sharing of electrons between two or more atoms. Ionic bonds form when two or more ions come together and are held together by charge differences.
Answer:
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
Ionic bond refers to a type of chemical bond which generates two oppositely charged ions. This bonding refers to the complete transfer of valence electrons between atoms
( Refer the attached image for their respective examples )

help!! help!! help!! help!! help!! help!! help!! help!! help!! explain the bond formation of ocl2 molecule
Answers
Answer:
it is formed by covalent bonds. the oxygen atom shares one elctron with each chlorine atom, forming single covalent bonds ( cl — o — cl )
The density was of a solution of sulfuric acid is 1.285g/cm3 and it is 38.08% (by weight) acid. How many millimeters of the acid solution do you need to supply 125 grams of sulfuric acid?
Answers
Answer:
255.5cm³
Explanation:
A solution that is 38.08 % by weight has 38.08g of sulfuric acid per 100g of total solution. Thus, mass of solution you need to obtain 125g is:
125g H₂SO₄ × (100g solution / 38.08g H₂SO₄) = 328.3g of solution
As density of the solution is 1.285g / cm³, the volume of 328.3g is:
328.3g × (1cm³ / 1.285g) = 255.5cm³
does tin or lead have a larger atomic radius??
Answers
Choose the best Lewis structure for a) OCl2 Shape of the molecule Hybridization of central atom b) CH2Cl2 Shape of the molecule Hybridization of central atom ICl5 Shape of the molecule Hybridization of central atom: d) SF4 Shape of the molecule Hybridization of central atom: e) BF3 Shape of the molecule Hybridization of central atom:'
Answers
The way that we represent a compound on the paper is that we have to draw the Lewis structure of the compound. This is the structure that can show the way that the atoms are connected including the kind of bonds and also the non bonding electrons that can be seen in the compound as we have it here.
We have to look out for the kind off orbital hybridization that has taken place in each one of the compounds that are shown in the 1question and this would now give us a clue as to what the accurate shape of the molecule that is under consideration should be here.
Learn more about Lewis Structure:brainly.com/question/20300458
#SPJ1
what caused the newt population to become more poisonous
Answers
Answer:
claim
Explanation:
CLAIM: The newt population became more poisonous because the snakes in this environment caused poison to be an adaptive trait, and Poison Level 10 is the most common because the newts with this trait were able to live longer and reproduce more than other newts.
How did we realize there were so many different elements?
Answers
answer:
science!
scientists discovered the table of periodic elements, and over time they discovered more and more, so thats how we know about so many different ones!
explanation:
hope this helped <3 also if wouldn't mind could you pls give me brainliest? (im trying to level up) thanks! :)
the distribution of electrons among orbitals in a many electron atom is known as its electron
Answers
The distribution of electrons among orbitals in a many-electron atom is known as its electron configuration.
The electron configuration describes how electrons are distributed in the various energy levels and orbitals around the nucleus of an atom. It is represented using a series of numbers and letters that indicate the principal energy level (n), the type of orbital (s, p, d, f), and the number of electrons in each orbital.
For example, the electron configuration for carbon (C) is 1s^2 2s^2 2p^2, which shows that carbon has two electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbital.
The electron configuration provides important information about the arrangement and organization of electrons within an atom, which in turn affects the atom's chemical properties and reactivity.
To learn more about electron configuration, visit:
https://brainly.com/question/29184975
#SPJ11
What mass of oxygen reacts with 9.2g Sodium? 4 Na + O₂ → 2 Na₂O
Answers
Answer:
About 3.201g O2
Explanation:

carbon monoxide (co) and hydrogen (h2) are fed to a continuous catalytic reactor operating at steady state. there are no other components in the feed. the outlet stream contains unconverted co and h2, along with the products methanol (ch30h), ethanol (c2hsoh), isopropanol (c3h70h), and carbon dioxide (
Answers
According to the information provided, a reaction between carbon monoxide, hydrogen and the creation of methanol, ethanol isopropanol and carbon dioxide is what happens in a continuous catalytic reactor.
Thus, Based simply on the provided information, it is impossible to pinpoint the precise reaction route and circumstances.
The Fischer-Tropsch synthesis, also known as the hydrogenation of carbon monoxide, is one conceivable process that could take place in this environment. In this process, carbon monoxide and hydrogen are transformed into a variety of hydrocarbons and oxygenates.
The following is a representation of the Fischer-Tropsch synthesis. H₂O stands for water produced as a byproduct, and n denotes the number of carbon atoms in the hydrocarbon compounds.
Thus, According to the information provided, a reaction between carbon monoxide, hydrogen and the creation of methanol, ethanol isopropanol and carbon dioxide is what happens in a continuous catalytic reactor.
Learn more about Fisher reaction, refer to the link:
https://brainly.com/question/32464208
#SPJ4
Which agricultural practices result in methane emission? Select the two correct answers.a. clearing land for farmsb. refrigerationc. manure management techniquesd. rice cultivation
Answers
The agricultural practices that results in methane emission is manure management. That is option C.
What is methane gas emission?Methane gas emission is defined as the release of large quantities of methane gas into the atmospheric region of the earth by production and transport of coal, natural gas, and oil, livestock and other agricultural practices, land use and by the decay of organic waste in municipal solid waste landfills.
The negative effects of the continuous emission of methane gas into the atmosphere would lead to global warming as it is one of the major greenhouse gases.
Manure management is one of the agricultural practices that can lead to the emission of methane gases.
Learn more about gases here:
https://brainly.com/question/25736513
#SPJ1
In order to have a more observable reaction, a student decides to add some deionized water to the Erlenmeyer flask. How will the experimental [HCl] be skewed
Answers
Adding deionized water to the Erlenmeyer flask will dilute the concentration of HCl in the solution. When deionized water is added to the flask, it increases the volume of the solution without changing the amount of HCl present. As a result, the concentration of HCl will decrease.
The concentration of a solution is defined as the amount of solute (in this case, HCl) dissolved in a given volume of solvent (in this case, deionized water). By adding more deionized water, the total volume of the solution increases, but the amount of HCl remains the same. Therefore, the concentration of HCl will decrease.
The effect of dilution on the concentration of a solution is described by the equation C1V1 = C2V2, where C1 and V1 represent the initial concentration and volume, and C2 and V2 represent the final concentration and volume. In this case, the initial concentration of HCl will be higher before adding deionized water, and the final concentration will be lower after adding deionized water.
In conclusion, adding deionized water to the Erlenmeyer flask will decrease the concentration of HCl, resulting in a skewed experimental [HCl].
To know more about HCl refer to:
https://brainly.com/question/27576431
#SPJ11
Science is divided in various fields of study except:
Answers
Science is divided into various fields of study except for pseudoscience which is not based on the scientific method.
What is science?Science refers to the total body of knowledge obtained by applying the scientific method.
Science can be divided into physical sciences, mathematics, life sciences, and social sciences.
In conclusion, science is divided into various fields of study except for pseudoscience which is not based on the scientific method.
Learn more about science here:
https://brainly.com/question/17216882
#SPJ1
Which element has four times as many protons in its nucleus than are found in neon.
Answers
Answer:
beryllium atom
Explanation:
An atom with two protons is always a helium atom. If scientists count four protons in an atom, they know it's a beryllium atom.
Please help me I am so confused. :)

Answers
The diagram model of emission lines four element could be the part of unknown star composition is C and D
Emission lines refer to the fact that glowing hot gas emits lines of light, whereas absorption lines refer to the tendency of cool atmospheric gas to absorb the same lines of light and when light passes through gas in the atmosphere some of the light at particular wavelengths is scattered resulting in darker bands
Here in the given data is unknown star and in that unknown element we have to chose which four element are match or seen as like unknown given sample so in the option the option c and d are as like as unknown sample because in the unknown sample the emission lines are same as in option c and d
Know more about emission lines
https://brainly.com/question/26672435
#SPJ1
A mass of 33.6g of magnesium carbonate, MgCO3, completely decomposed when it was heated. It made 16.0 g of magnesium oxide, MgO Calculate the mass of carbon dioxide, CO2, produced in this reaction.
Answers
Answer:
17.6g
hahahahahahhaha
This diagram shows a plant cell. Give the name of Part A

Answers
Answer:
A part is called Chloroplasts
Please discuss the following items in your introduction:
• Law of Conservation of Mass (with example using an equation)
• Reactants/ products
• Word equations/ skeleton/ balanced chemical equations
Answers
Law of conservation of mass states that in a closed system, the mass would be the same.
In a reaction, the compounds’ undergoing reaction is called reactants and the resultant of the reaction is called products.
Word equation uses words instead of chemical formulas. The skeleton equations use chemical formulas without mentioning the state of the compounds or the concentration. In balanced chemical reaction, mass and charge would be balanced.
Law of conservation of mass is similar to law of conservation of energy. It says that in a closed system, the mass would remain the same as mass is neither created nor destroyed.
\(H_{2}O\) → \(H_{2} + O_{2}\)
In this reaction, \(H_{2}O\) is the reactant which has a mass of 18. H₂ and O₂ are the products with mass of 2 and 16 respectively.
In word equations, equation 1 can be written as
Water → Hydrogen + Water
In skeleton equation,
H₂0 → H₂ + O₂
In balanced chemical reaction,
2 H₂0 → 2 H₂ + O₂
To know more about Word equation
https://brainly.com/question/15423243
#SPJ1
What mass of H2 forms when
35.25 g of Al reacts with excess
hydrochloric acid?
2AI+ 6HCI→ 2AlCl3 + 3H₂
Al: 26.98 g/mol
H₂: 2.02 g/mol
[?] g H₂

Answers
35.25/26.98 (mass reacted/molar mass of Al)
2) Find number of moles of H2 produced:
Number of mol. of Al x 3/2 (based on stoichiometry)
3) Find mass of H2 formed:
Number of mol. of H2 x molar mass of H2 = 3.9588mol = 4 mol (estimated value)
Consider the following reaction: 2CH3OH(g)→2CH4(g)+O2(g),ΔH=+252.8 kJ Part A:Calculate the amount of heat transferred when 29.0 g of CH3OH(g) is decomposed by this reaction at constant pressure. Express the heat to three significant digits with the appropriate units. part B: For a given sample of CH3OH, the enthalpy change during the reaction is 82.3 kJ . What mass of methane gas is produced? Express the mass to three significant digits with the appropriate units Part C :How many kilojoules of heat are released when 38.5 g of CH4(g) reacts completely with O2(g) to form CH3OH(g) at constant pressure? Express heat to three significant digits with the appropriate units
Answers
A. The amount of heat transferred when 29.0 g of CH3OH(g) is decomposed by this reaction at constant pressure is 114 kJ.
B. The mass of methane gas produced is 8.67 g.
C. The amount of heat released when 38.5 g of CH4(g) reacts completely with O2(g) to form CH3OH(g) at constant pressure is 238 kJ.
A. How to calculate the amount of heat transferred ?The molar mass of CH3OH is 32.04 g/mol. Therefore, 29.0 g of CH3OH is equal to 29.0 g / 32.04 g/mol = 0.904 mol of CH3OH.
From the balanced equation, the reaction produces 2 moles of CH4 and 1 mole of O2 for every 2 moles of CH3OH decomposed.
Therefore, the amount of heat transferred when 0.904 mol of CH3OH is decomposed is:
0.904 mol CH3OH × (252.8 kJ / 2 mol CH3OH) = 114 kJ
So, the amount of heat transferred when 29.0 g of CH3OH(g) is decomposed by this reaction at constant pressure is 114 kJ.
B. How to calculate the mass of methane gas produced when 82.3 kJ of heat is transferred in the given chemical reaction?The enthalpy change during the reaction is 82.3 kJ for a given sample of CH3OH. We need to find the mass of methane gas produced.
From the balanced equation, 2 moles of CH3OH produces 2 moles of CH4.
Therefore, the amount of CH4 produced when 82.3 kJ of heat is transferred is:
2 mol CH4 × (82.3 kJ / (252.8 kJ / 2 mol CH3OH)) = 0.540 mol CH4
The molar mass of CH4 is 16.04 g/mol. Therefore, the mass of CH4 produced is:
0.540 mol CH4 × 16.04 g/mol = 8.67 g
So, the mass of methane gas produced is 8.67 g.
C. How to calculate the amount of heat released when 38.5 g of CH4 reacts completely with O2 to form CH3OH at constant pressure?From the balanced equation, 1 mole of CH4 produces 1 mole of CH3OH when reacted with O2.
Therefore, the amount of heat released when 38.5 g of CH4 reacts completely with O2 to form CH3OH is:
38.5 g CH4 / 16.04 g/mol CH4 × (252.8 kJ / 2 mol CH3OH) = 238 kJ
So, the amount of heat released when 38.5 g of CH4(g) reacts completely with O2(g) to form CH3OH(g) at constant pressure is 238 kJ.
Learn more about heat transferred
brainly.com/question/13433948
#SPJ11
What volume of 17.8 M stock sulfuric acid solution would be needed to make 2.0L of 0.200 M diluted sulfuric acid solution?
Answers
Answer:
Required Volume is 22.47ml
Explanation:
To make 2.0L of 0.2M sulfuric acid required moles = 0.2 x 2 = 0.4 moles
To get 0.4 from 17.8M required quantity = 1000/17.8 x 0.4 = 22.47ml
A. The pendulum would accelerate to the left
B. The pendulum would accelerate downward
C. The pendulum would accelerate to the right
D. The pendulum would accelerate upward
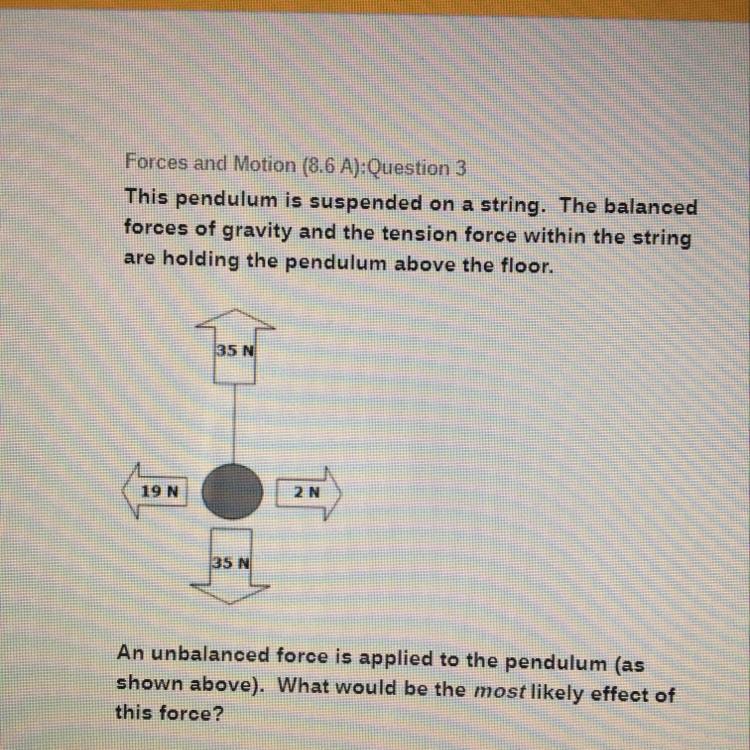
Answers
Answer:
The pendulum would accelerate to the left
Explanation:
hope this helps
describe what the isotopes of an element have in common and how are they different.
Answers
Answer:
Isotopes of an element are atoms of the same element that have different number of neutrons.

Is sodium ion or sodium metal in table salt?
Answers
Answer:
sodium ion
Explanation:
Pinacolone: __ml; 0.50g 0.00499__ mol piperonal: 0,75 g 0,005 mol KOH: 0.3 0.00535 __mol Limiting Reagent: _Piperond. Product: Pinacolone-Piperonal Adduct 0.43 melting Point: Description of product appearance:_tihe gole Yellow Deedles Data Analysis: Calculate the Theoretical Yield (show calculation): Pinacolone-Piperonal Adduct Theoretical Yield: mol; Calculate the Percent Yield (show calculation): Actual Yield: - mol Percent Yield:
Answers
mol Percent Yield 47.25%.
Full calculation in image
To know more about Percent Yield visit:
https://brainly.com/question/17042787
#SPJ4

Use the food chain to answer the questions.1.Label each part of the food chain with the following terms.____________________________________________________2. Which organism has the most energy? ________________________3. Which organism is a herbivore? ________________________4. Which organism is a carnivore? _______
Answers
label each part of the food chain with the following terms.
Sun → Grass → Rabbit → Fox
Producer → Primary Consumer → Secondary Consumer → Tertiary Consumer
Which living thing possesses the most energy?As the main energy source for the food chain, the sun has the most energy.
Which organism consumes only plants?Given that it consumes grass, a plant and the top producer in the food chain, the rabbit is a herbivore.
What type of organism consumes meat?The rabbit, a herbivore and the first consumer in the food chain is what the fox eats, making it a carnivore.
In a food chain, the sun supplies the energy needed for plants to employ photosynthesis to produce their own food. These plants are at the base of the food chain and are referred to as producers.
Carnivores, also known as secondary and tertiary consumers, consume other animals in the food chain while herbivores, or primary consumers, eat plants.
The principal source of energy and designated producer in the given case is the sun. The term "primary consumer" refers to grass, which is the first consumer in the food chain and consumes sunlight.
The fox, which feeds on the rabbit, is classified as the tertiary consumer, while the rabbit, which eats grass, is classified as the secondary consumer.
learn more about the food chain here
https://brainly.com/question/2179
#SPJ1
6. (20 pts) Provide a synthetic route to prepare the target molecule using the malonic ester synthesis. All the carbon in the product must derive from the given starting materials, but you can use any other necessary reagents.
Answers
Answer: Hello some data related to your question is missing attached below is the missing data
answer : attached below is the detailed solution ( synthetic route
Explanation:
Attached below is the synthetic route for the preparation of the target molecule and
we will perform a coupling reaction to get the Final product as seen in the question

