2KClO3-->2KCl+3O2
How many grams of potassium chloride (KCl) are produced from 45.0 g KClO3?
Answers
27.36 grams of potassium chloride (KCl) are produced from 45.0 g KClO3.
What is potassium chloride?Potassium chloride is also known as potassium salt and is used as a medication to treat and prevent low blood potassium.
The balanced chemical equation is:
2KClO3 → 2KCl + 3O2
Molar mass of KClO3 is:
KClO3 = 39.0983 g/mol (K) + 35.453 g/mol (Cl) + 3(15.9994 g/mol) (O)
= 122.5503 g/mol
Number of moles of KClO3 is: 45.0 g / 122.5503 g/mol = 0.3672 mol
As 2 moles of KClO3 produce 2 moles of KCl, number of moles of KCl produced is also 0.3672 mol.
KCl = 39.0983 g/mol (K) + 35.453 g/mol (Cl)
= 74.5513 g/mol
Mass of KCl produced is: 0.3672 mol × 74.5513 g/mol = 27.36 g
Therefore, 27.36 grams of potassium chloride (KCl) are produced from 45.0 g KClO3.
To know more about potassium chloride, refer
https://brainly.com/question/25380525
#SPJ1
Related Questions
Can you use sugar instead of salt?.
Answers
We are unable to replace salt with sugar. Many soluble carbohydrates with a sweet flavour and that are used in food are collectively referred to as sugar.
In addition to fructose, simple sugars also go by the name monosaccharides. also galactose. Disaccharides or double sugars are other names for compound of carbohydrates. Rock salt, also known as carbohydrates, is a naturally occurring crystalline mineral that is made up mostly of sodium chloride (NaCl), a chemical molecule that is a member of the larger class of salts. In seawater, salt is found in enormous amounts.
Seawater, where it makes up the majority of the minerals, contains a significant amount of it. Animal life cannot exist without salt, and humans' basic tastes include saltiness.
Learn more about carbohydrates here
https://brainly.com/question/14878446
#SPJ4
What is the mass number of a carbon atom which has 7 neutrons?
Answers
Answer:
Carbon-13
Explanation:
Carbon have three isotopes. Isotopes are the atoms of the same element which has a different number of neutrons. Carbon has 3 isotopes.
Carbon-12 : 6 electrons I 6 protons I 6 neutrons
Carbon-13 : 6 electrons I 6 protons I 7 neutrons
Carbon-14 : 6 electrons I 6 protons I 8 neutrons
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane,.
Answers
Answer:
2C2H6(g)+7O2(g)→4CO2(g)+6H2O(l)
Explanation:
When ethane is burned in presence of oxygen, it produces carbon dioxide (CO_2)(CO2) and water (H_2O)(H2O) .
According to law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of reactants must be equal to the mass of products.
In order to get the same mass on both sides, the number of atoms of each element on product side must be same as the number of atoms of each element on reactant side.Thus the equations are balanced.
2C_2H_6(g)+7O2(g)\rightarrow 4CO_2(g)+6H_2O(l)2C2H6(g)+7O2(g)→4CO2(g)+6H2O(l)
A pure substance __________ . must be an element. can be separated into simpler substances by physical means. has different chemical and physical properties depending on its source. can be separated into simpler substances by chemical means.
Answers
Answer:
a pure substance has different chemical and physical properties depending on its source
Impurity atoms in ceramic materials may form substitutional and interstitial solid solutions (T/F).
Answers
Impurity atoms in ceramic materials may form substitutional and interstitial solid solutions is True.
Ceramic materials are an inorganic compound of metals and nonmetals. Impurities in ceramic materials can be intentionally added to improve the properties of the materials. The impurities in the ceramic materials may form substitutional and interstitial solid solutions.
Substitutional solid solution refers to a type of solid solution in which the impurity atoms substitute the host atoms of the crystal lattice while retaining the crystal structure. Interstitial solid solution refers to a type of solid solution in which impurity atoms occupy the interstitial sites in the crystal lattice of the ceramic material, but the crystal structure changes slightly due to the presence of impurity atoms.
The concentration of impurities can vary up to 150 ppm (parts per million).
Therefore, the statement "Impurity atoms in ceramic materials may form substitutional and interstitial solid solutions" is True.
Learn more about interstitial solid in the link:
https://brainly.com/question/30836773
#SPJ11
The structures of four organic compounds are shown below. Which statement is not correct?
A. Only one of the compounds is an alcohol.
B. Only one of the compounds is an alkane .
C. Only one of the compounds is unsaturated
D. Only three of the compounds are hydrocarbons.

Answers
Answer:
Option B. Only one of the compounds is an alkane
Explanation:
To know which option is wrong us, we simply determine the class to which each diagram belongs.
Diagram 1:
CH4 is called methane and it is a member of the alkane series which are saturated hydrocarbon.
Diagram 2:
CH3CH3 is called ethane and it is a member of the alkane series which are saturated hydrocarbon.
Diameter 3:
CH2=CH2 is called ethene and it is a member alkene series which are unsaturated hydrocarbon.
Diagram 4:
CH3CH2OH is called ethanol which is a member of the alcohol family. CH3CH2OH is also a saturated alcohol as it only contains single bonds.
From the above, we can see that:
1. Only one compound is alcohol i.e CH3CH2OH.
2. There are two members of the alkane series i.e CH4 and CH3CH3.
3. Only one compound is unsaturated i.e CH2=CH2. Unsaturated compound has one or more double bond.
4. Only three compounds are hydrocarbon i.e CH4, CH3CH3 and CH2=CH2. Hydrocarbons are compounds containg only carbon and hydrogen.
Therefore, option B is wrong as there are two members of the alkane present.
PLEASE ANSWER WITH EXPLANATION
Val cut a cold apple into slices, placed the slices on a plate, and left the plate on the kitchen counter. Over the next two hours, Val observed that the apple slices started to dry out and turn brown. She also noted that the apple slices warmed to room temperature, and a couple of fruit flies started to fly around them. Which of the following would be a good Claim and Evidence explaining if a chemical or physical change occurred?
A. This is an example of a chemical change. My evidence that supports this claim is that the apple is room temperature.
B. This is an example of a chemical change. My evidence that supports this claim is that the apple changed color.
C. This is an example of a physical change. My evidence that supports this claim is that the apple changed color.
D. This is an example of a physical change. My evidence that supports this claim is that the apple is room temperature.
Answers
Answer:B
Explanation: A chemical change is something that is irreversible
Answer:
The answer is D
Explanation:
The answer is D because the apple went from being cold to the temperature of the room. No chemicals or anything were done to the apples. They were just set on the counter so this is a physical change. The apples turning room temperature was the cause of the apples turning brown, so the best answer is D.
For strong electrolytes, i = number of per mole of solute dissolved. CaCl dissolves yielding three ions, one Ca ion and two Clions, thus i = (NH. ),P dissolves yielding four ions, three NH' ions and one Pion, thus i = "Colligative Properties Study Guide" by Montgomery College is licensed under CC BY 4. 0
Answers
The statement you provided refers to the determination of the van't Hoff factor (i) for strong electrolytes. The van't Hoff factor represents the number of ions produced per mole of solute dissolved in a solution.
For example, when calcium chloride (CaCl2) dissolves, it dissociates into three ions: one Ca2+ ion and two Cl- ions. Therefore, the van't Hoff factor (i) for CaCl2 is 3 because it produces three ions per mole of solute dissolved.
Similarly, when ammonium phosphate (NH4)3PO4 dissolves, it dissociates into four ions: three NH4+ ions and one PO43- ion. Thus, the van't Hoff factor (i) for (NH4)3PO4 is 4 because it yields four ions per mole of solute dissolved.
The van't Hoff factor is essential in various calculations related to colligative properties, such as boiling point elevation and freezing point depression, where it is used to account for the number of particles in solution.
learn more about electrolytes here
https://brainly.com/question/32477009
#SPJ11
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
O
0
0
Name:
Chemical formula:
Answers
Chemical formula:O3 (write the 3 as subscript)
The photos below show four pairs of objects.
Person and dog
Person and Earth
Person and building
Person and ball
Which pair of objects is experiencing the greatest gravitational force?
A. The person and the ball
B. The person and Earth
C. The person and the dog
D. The person and the building

Answers
Answer:
person and earth since they are closer to the core ? maybe
What is HYDROGEN and OXYGEN?
Answers
Answer:
Hydrogen is the first element in the periodic table. Oxygen is the eighth element in the periodic table.
Both elements are gases.
Hydrogen is used for the Haber process and rocket fuel.
Oxygen is used for breathing and production purposes.
Air composes 21% oxygen and 0.0001% hydrogen.
Water is an example that contains both hydrogen H2 and oxygen O2 molecules.
Answer:
Hydrogen is the chemical element with the Symbol H and atomic number 1.
Hydrogen is the lightest element in the periodic table and it is the most abundant in the universe.
WHILE:
Oxygen is the chemical element with the symbol O and the atomic number 8.
It is a member of the chalcogen group in the periodic table.
This is what we humans take in to breathe that gives us life.
Describe how the charges of the subatomic particles interact with one another.
Answers
Answer:
Electrons, Protons, and Neutrons. ... Protons are another type of subatomic particle found in atoms. They have a positive charge so they are attracted to negative objects and repelled from positive objects. Again, this means that protons repel each other
How many molecules are in 48.0 grams of NaOH?
Answers
Hope this helped :)
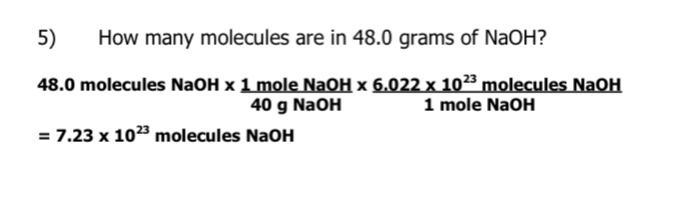
What is the empirical formula for propane?
Answers
Answer:
The empirical formula for propane is C3H8.
Answer:
Because propane is made up of only hydrogen and carbon _ the chemical formula is C3H8..
mass of 2 into 10 to power 21 number of atoms of an element is 0.4 gram what is the mass of 0.5 mole of the elements
Answers
The mass of 0.5 mole of the element is approximately 6.025 grams.
To calculate the mass of 0.5 mole of the element, we need to know the molar mass of the element.
Given that the mass of 2 x 10^21 atoms of the element is 0.4 grams, we can use this information to find the molar mass.
The number of atoms in 1 mole of any substance is given by Avogadro's number, which is approximately 6.022 x 10^23 atoms/mol.
First, we calculate the molar mass of the element using the given information:
Molar mass = Mass of 2 x 10^21 atoms / Number of moles of 2 x 10^21 atoms
Molar mass = 0.4 g / (2 x 10^21 atoms / (6.022 x 10^23 atoms/mol))
Molar mass ≈ 0.4 g / (3.32 x 10^-2 mol)
Molar mass ≈ 12.05 g/mol
Now that we know the molar mass of the element is approximately 12.05 g/mol, we can calculate the mass of 0.5 mole of the element:
Mass = Molar mass x Number of moles
Mass = 12.05 g/mol x 0.5 mol
Mass = 6.025 grams
for more such questions on element
https://brainly.com/question/28376204
#SPJ8
A syringe has 15 mL of gas at 3 atm of pressure. After squeezing the syringe to 7mL, what is the new pressure?
Answers
Temperature constant, Boyle's law
P₁V₁=P₂V₂
3.15 = P₂.7
P₂=6.43 atm
The main purpose of homeostasis is to
A. fight disease-causing organisms.
B. keep internal conditions stable.
C. produce offspring.
D. replicate DNA.
Answers
The average atomic mass of nickel is 58.69 amu. It has 28 protons. How many electrons does nickel have
Answers
Answer:
Nickel has a total of 28 electrons.
Explanation:
Identify any incorrect formulas. Explain your answer.
a. Mg2(SO4)3
b. Rb3As
c. BeCl3
d. NaF
Wish I could award more points but I'm running low.
Answers
The formula of a compound is written based on the valency of each bonded atoms. The formulas Mg₂(SO₄)₃, Rb₃As are incorrect. Such compounds does not exist.
What is chemical formula?The formula of a chemical compound is written based on the number of atoms participated in bonding. Magnesium is second group element having two valence electrons.
Magnesium thus donates its two electrons into the bonding atom or group. The sulphate group SO₄ have a valency of 2 it gains two electrons from Mg when bonded.
Thus the formula of magnesium sulphate being MgSO₄ and not Mg₂(SO₄)₃. Similarly Rb is a second group metal having two valence electrons. Its valency being two. As is a halogen with valency one. Thus they cannot form a compound like Rb₃As.
Hence, option a and b are incorrect formulas.
To find more about chemical formulas, refer the link below:
https://brainly.com/question/11995171
#SPJ2
what is the method use to separate sand and water
Answers
Sand and water can be separated by any of the following methods:
1. Sedimentation and decantation: This method involves the mixture being kept undisturbed for some time. After some time, sand being heavier and insoluble in water, settles down at the bottom of container. Now, water is poured into another container to separate it from sand.
2. Filtration: This method involves the mixture being passed through a filter paper (a filter with very fine pores). Sand particles being larger in size are retained by the filter paper and get separated from water.
I hope this helps! :D
Coefficients are used in a chemical formula to show the number of each element. True or False
Answers
Answer:
The same number of each element present before the reaction takes place must also be present on the product side of the equation. Coefficients are placed in front of a chemical formula to show the number of moles of that substances that are necessary for the reaction to occur.
Explanation:
Dihydrogen monoxide is a(n) ____. A. covalent compound B. molecular formula C. empirical formula D. Ionic compound
Answers
Answer:
D. Ionic compound
Explanation:
Just based on my opinion
Correct me if I'm wrong tnx:<
Dihydrogen monoxide is a covalent compound. Option A is correct.
Covalent compounds are compounds that are held together by covalent bonds. Covalent bonds are formed when two atoms share electrons. In dihydrogen monoxide, the two hydrogen atoms share a pair of electrons with the oxygen atom. This forms a molecule of water, which is a covalent compound.
Ionic compounds are compounds that are held together by ionic bonds. Ionic bonds are formed when one atom donates electrons to another atom. In an ionic compound, the electrons are not shared, but are transferred from one atom to another. The molecular formula for dihydrogen monoxide is H₂O.
The difference between a molecular formula and an empirical formula is that the molecular formula shows the actual number of atoms of each element in a molecule, while the empirical formula shows the simplest ratio of the atoms of each element in a molecule. Option A is correct.
To know more about the Compound, here
https://brainly.com/question/29448163
#SPJ2
Why is it important for scientists to publish a
description of their procedures along with the results
of their experiments?
Answers
Answer:
Anything related to science needs to be detailed and understood by a range of people. Or, what they published may not create any impact if you can't read it and understand what you just read.
Explanation:
True/False?pyrophoric substances can burn spontaneously in air.
Answers
The given statement "pyrophoric substances can burn spontaneously in air." is true as pyrophoric materials are those that spontaneously ignite when ignited and exposed to air.
A chemical that spontaneously ignites in air at a temperature of 130 °F (54.4 °C) or lower is referred to as a pyrophoric gas. Chemicals classified as pyrophoric will spontaneously ignite in air at 130°F (54.4°C) or lower. Pyrophores frequently burn up in the presence of air due to a strong reaction with oxygen or water vapor.
Pyrophoric materials are those that spontaneously ignite when ignited and exposed to air. A brief overview of pyrophoric materials and flame safety is provided here. Materials classified as pyrophoric ignite instantly when exposed to oxygen. They may also react with water, producing heat and hydrogen (a flammable gas).
Learn more about pyrophoric substances at:
brainly.com/question/30399045
#SPJ4
4. Why does ammonia, NH3, behave as a base when it reacts with an acid?
A It accepts a neutron and becomes NH3+.
B It accepts a proton and becomes NH4+.
OC It donates a proton and becomes NH2
Answers
B is the answer ,ammonium accepts a proton and becomes ammonium ion
what is the minimum amount of 6.0 m necessary to produce 25.0 g of according to the reaction between aluminum and sulfuric acid?
Answers
The amount of 6M H₂SO₄ to produce 25 grams of H₂ is 2.08 L.
The reaction of aluminum and sulfuric acid is,
2Al + 3H₂SO₄ = Al₂(SO₄)₃ + 3H₂
So, as we can see, from the reaction,
3 moles of H₂SO₄ produces 3 moles of H₂.
So, we can write,
Moles of H₂SO₄ = moles of H₂
Moles = molarity × volume
Moles = Given mass/molar mass
(Molarity × volume) of H₂SO₄ = Given mass of H₂/Molar mass of H₂
Molarity of H₂SO₄ is given to be 6M and and given mass lf H₂ is 25 g. So, putting values,
6×volume = 25/2
Volume = 2.08 L.
So, the amount of required H₂SO₄ is 2.08 L.
To know more about Moles, visit,
https://brainly.com/question/15356425
#SPJ4
Complete Question - What is the minimum amount of 6.0 M H2SO4 necessary to produce 25.0 g of H2(g) according to the reaction between aluminum and sulfuric acid? 2 Al(s) + 3 H2SO4(aq)-Al2(SO4)3(aq) + 3 H2(g)
comparison of methods for spectral alignment and signal modelling of gaba‐edited mr spectroscopy data
Answers
Comparison of techniques for GABA-edited MR spectroscopy data spectral alignment and signal modeling.
There are many ways to align and quantify magnetic resonance spectroscopy (MRS) data in order to assess -aminobutyric acid in vivo (GABA). Because there are few ground-truth measurements available, there is little research comparing the performance of different approaches. In comparison to white matter, the concentration of GABA is almost twice as high in grey matter.
Here, we assess the performance of four spectral alignment approaches (i.e., retrospective frequency and phase drift correction) and six GABA signal modeling methods using the percentage of grey matter present in the MRS voxel as a proxy for ground-truth GABA concentration.
The alignment to the creatine (Cr) signal generates GABA+ estimates that account for around twice as much of the diversity in grey matter as the next highest performing alignment approach, according to our analysis of a broad dataset of 432 MEGA-PRESS scans targeting several brain areas.
Additionally, Cr alignment produced the fewest outliers and was the strongest. As opposed to this, all signal modeling techniques—aside from the single-Lorentzian model—performed equally well.
Our findings indicate that rather than frequency originating from first-order phase offsets within subspectra, performance variability is predominantly caused by variances in the zero-order phase calculated by each alignment method.
Learn more about spectroscopy here:
https://brainly.com/question/14854785
#SPJ4
Energy used for metabolism comes primarily from which biomolecules a) Polysaccharides only b) Lipid only c) Lipid and Carbohydrates D) amino acids
Answers
Answer:
C) Lipids and Carbohydrates
Explanation:
Lipids, or fats, are stored long-term for energy when an organism cannot find carbohydrates (specifically glucose), which are used as short-term energy.
Pu-238 (Plutonium, 238) decays by α emission to form an atom, which atom is this?
Answers
Answer:
Helium atom
Explanation:
For the RTGs flown by the United States, the radioisotope has been some chemical form of plutonium-238 (238Pu or Pu-238). Plutonium-238 decays primarily by the emission of an alpha particle (a helium atom without its electrons).