5. Scientists always develop a plan when they try to learn something about our natural world. Which
sequence correctly shows the steps scientists follow in their plan?
A. make observations, develop an idea, obtain evidence, suggest an explanation
B. obtain evidence, suggest an explanation, develop an idea, make observations
C. suggest an explanation, obtain evidence, make observations, develop an idea
D. develop an idea, suggest an explanation, obtain evidence, make observations
Answers
Related Questions
What is the equilibrium constant equation for the chemical process below?
2A + B2 —> A₂B2
Answers
Let's see
\(\\ \rm\dashrightarrow k=\dfrac{Concentration\:of\: products}{Concentration\:of\: reactants}\)
\(\\ \rm\dashrightarrow k=\dfrac{\left[A_2B_2\right]}{\left[A\right]^2\left[B\right]}\)
What would happen on the Earth if the Sun’s radiation could not travel through space?
Answers
Answer:
We'd all freaking freeze to death and be standing there like the black beetles trend.
Explanation:
This is a joke, please don't take this seriously.
Propose an efficient synthesis for the following compound (0.4 points) 2-methylpentan-3-one
Answers
To synthesize 2-methylpentan-3-one efficiently, following process has to be followed:
Start with the synthesis of 2-methylpentan-3-ol, which can be prepared via a Grignard reaction. Firstly, prepare 2-methylpentan-3-ol by reacting ethyl magnesium bromide with 3-methylbutanal.
Afterward, hydrolysis of this Grignard reagent using HCl or dilute sulfuric acid provides the alcohol.2-methylpentan-3-ol.
( Grignard reaction -The Grignard reaction is an organometallic chemical reaction in which carbon alkyl, allyl, vinyl, or aryl magnesium halides (Grignard reagent) are added to the carbonyl groups of either an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds.)
Now,the oxidation of 2-methylpentan-3-ol is carried out using oxidizing agents like PCC (pyridinium chlorochromate), PDC (pyridinium dichromate), or Jones reagent to synthesize 2-methylpentan-3-one.Oxidation of 2-methylpentan-3-ol to synthesize 2-methylpentan-3-one can be represented as follows: PCC or PDC or Jones Reagent2-methylpentan-3-one.
To know more about Grignard reaction visit:
https://brainly.com/question/31845163
#SPJ11
If a sample of iron with a density of 7.80 g/cm3 displaces 75.0 ml of water when placed in a beaker, what is its mass? 585 g 9.62 g 0.104 g
Answers
The mass of iron sample will be 585 grams.
According to the question,
Density of iron sample = 7.80 g/cm³
Volume of water displaced = 75.0 ml
1 ml = 1 cm³
Therefore, 75 ml = 75 cm³
Density is defined as the mass (m) of a substance per unit volume (V). It can be generally denoted by ρ. Therefore, ρ = m/V.
Mass is the amount of matter present in a substance. Volume is the 3-D space occupied by a substance.
In the given question density and volume are provided and to find the value of mass, the formula can be rewritten as: m = ρ × V.
Hence, m = 7.80 g/cm³ × 75 cm³
m = 585 grams.
To know more about density, here
brainly.com/question/15164682
#SPJ4
What hazard does the symbol containing the hand represent?
chemical hazard
heat hazard
biohazard
radioactive hazard
Answers
Answer:
chemical hazard
Explanation:
Can you guys please help me with this chart PLEASEEE?

Answers

How much acetic acid (ch3cooh) in grams, would be required to make 1 liter of a 1.5 m acetic acid solution in water?
Answers
The amount of acetic acid (CH₃COOH) in grams needed to prepare the solution is 90
What is molarity?This is defined as the mole of solute per unit litre of solution. Mathematically, it can be expressed as:
Molarity = mole / Volume
How to determine the mole of CH₃COOHMolarity = 1.5 MVolume = 1 L Mole of CH₃COOH =?Molarity = mole / Volume
Cross multiply
Mole = Molarity × Volume
Mole of CH₃COOH = 1.5 × 1
Mole of CH₃COOH = 1.5 mole
How to determine the mass of CH₃COOHMole of CH₃COOH = 1.5 moleMolar mass of CH₃COOH = 60 g/mol Mass of CH₃COOH =?Mole = mass / molar mass
Cross multiply
Mass = mole × molar mass
Mass of CH₃COOH = 1.5 × 60
Mass of CH₃COOH = 90 g
Thus, 90 g of CH₃COOH is needed to prepare the solution
Learn more about molarity:
https://brainly.com/question/15370276
#SPJ4
Hypothesis: If a material undergoes a
chemical change, then it will not retain its
original properties because a new substance
is formed.
To test the hypothesis above, you will observe the
changes during the experiment.
To do this, you will use these observations to
compare the___
of the
substances
to the__
substances.
of the

Answers
Answer:
I hate to not answer and have you repost this if you could repost it with the choices by clicking the arrow I can figure it out a lot faster and I'll copy and paste to show you that it's right
Explanation:
I'm good with history biology sum math so if you want to do what I asked and reposted I can give you the answers and I will show that they are correct I won't just guess like some people do just to get points cuz I don't care about points I just get on here to help people
Answer: The answer for the blanks is initial appearance and than final appearance.
Explanation:
The standard heat of formation of sulfur dioxide is = Q kJ/mol. The standard heat of formation of sulfur trioxide is = R kJ/mol. What would be the ΔH for the reaction of two moles of sulfur dioxide with oxygen to produce two moles of sulfur trioxide?
Answers
The balanced equation for the reaction of two moles of sulfur dioxide with oxygen to produce two moles of sulfur trioxide is:
2 SO2(g) + O2(g) → 2 SO3(g)
What would be the ΔH for the reaction of two moles of sulfur dioxide with oxygen to produce two moles of sulfur trioxide?To determine the enthalpy change (ΔH) for this reaction, we can use Hess's law, which states that the overall enthalpy change for a reaction is equal to the sum of the enthalpy changes for a series of reactions that add up to the overall reaction. In other words, if we can find the enthalpy changes for a series of reactions that include the same reactants and products as the overall reaction, we can add them up to find the enthalpy change for the overall reaction.
To use Hess's law in this case, we can start with the given standard heats of formation and write the equation for the formation of sulfur trioxide from its constituent elements:
S(s) + 3/2 O2(g) → SO3(g) ΔHf° = R kJ/mol
Note that the enthalpy change for this reaction is equal to the standard heat of formation of sulfur trioxide (ΔHf°). Next, we can write the equation for the decomposition of sulfur dioxide into sulfur and oxygen:
2 SO2(g) → 2 S(s) + 2 O2(g) ΔH1
Note that this reaction is the reverse of the formation of sulfur dioxide from its constituent elements, so we need to reverse the sign of the enthalpy change (ΔH1) for that reaction. Finally, we can write the equation for the reaction we are interested in, which is the sum of the formation of sulfur trioxide and the decomposition of sulfur dioxide:
2 SO2(g) + O2(g) → 2 SO3(g) ΔH2
Using Hess's law, we can express the enthalpy change for the overall reaction (ΔH2) in terms of the enthalpy changes for the two component reactions:
ΔH2 = ΔHf° - ΔH1
Substituting the given values, we get:
ΔH2 = R kJ/mol - (-Q kJ/mol) = R + Q kJ/mol
Therefore, the enthalpy change for the reaction of two moles of sulfur dioxide with oxygen to produce two moles of sulfur trioxide is equal to the sum of the standard heats of formation of sulfur trioxide and sulfur dioxide, which is R + Q kJ/mol.
Learn more about enthalpy change from
https://brainly.com/question/27197408
#SPJ1
The balanced equation for the reaction of two moles of sulfur dioxide with oxygen to produce two moles of sulfur trioxide is:
2 SO2(g) + O2(g) → 2 SO3(g)
What would be the ΔH for the reaction of two moles of sulfur dioxide with oxygen to produce two moles of sulfur trioxide?To determine the enthalpy change (ΔH) for this reaction, we can use Hess's law, which states that the overall enthalpy change for a reaction is equal to the sum of the enthalpy changes for a series of reactions that add up to the overall reaction. In other words, if we can find the enthalpy changes for a series of reactions that include the same reactants and products as the overall reaction, we can add them up to find the enthalpy change for the overall reaction.
To use Hess's law in this case, we can start with the given standard heats of formation and write the equation for the formation of sulfur trioxide from its constituent elements:
S(s) + 3/2 O2(g) → SO3(g) ΔHf° = R kJ/mol
Note that the enthalpy change for this reaction is equal to the standard heat of formation of sulfur trioxide (ΔHf°). Next, we can write the equation for the decomposition of sulfur dioxide into sulfur and oxygen:
2 SO2(g) → 2 S(s) + 2 O2(g) ΔH1
Note that this reaction is the reverse of the formation of sulfur dioxide from its constituent elements, so we need to reverse the sign of the enthalpy change (ΔH1) for that reaction. Finally, we can write the equation for the reaction we are interested in, which is the sum of the formation of sulfur trioxide and the decomposition of sulfur dioxide:
2 SO2(g) + O2(g) → 2 SO3(g) ΔH2
Using Hess's law, we can express the enthalpy change for the overall reaction (ΔH2) in terms of the enthalpy changes for the two component reactions:
ΔH2 = ΔHf° - ΔH1
Substituting the given values, we get:
ΔH2 = R kJ/mol - (-Q kJ/mol) = R + Q kJ/mol
Therefore, the enthalpy change for the reaction of two moles of sulfur dioxide with oxygen to produce two moles of sulfur trioxide is equal to the sum of the standard heats of formation of sulfur trioxide and sulfur dioxide, which is R + Q kJ/mol.
Learn more about enthalpy change from
brainly.com/question/27197408
#SPJ1
floridium atoms (a hypothetical metal) are in a face-centered cubic unit cell, and the edge length of the unit cell is 310.2 pm. what is the atomic radius of floridium in pm to one decimal place?
Answers
The atomic radius of Floridium atoms in the FCC unit cell is approximately 109.8 pm to one decimal place.
The face-centered cubic unit cell contains 4 atoms. The edge length of the unit cell can be calculated using the formula:
a = 2 * (radius of atom)
Therefore, the radius of each floridium atom can be calculated as:
radius of atom = a / (2 * sqrt(2))
radius of atom = 310.2 pm / (2 * sqrt(2))
radius of atom = 109.9 pm
Therefore, the atomic radius of floridium is 109.9 pm to one decimal place.
To find the atomic radius of Floridium atoms in a face-centered cubic (FCC) unit cell, we can follow these steps:
1. Recall the relationship between the edge length (a) and the atomic radius (r) in an FCC unit cell: a = √2 * 4r.
2. Solve for the atomic radius (r) using the given edge length (a = 310.2 pm).
Step 1: Relationship between edge length and atomic radius in an FCC unit cell
For an FCC unit cell, the relationship between the edge length (a) and the atomic radius (r) is given by the formula:
a = √2 * 4r
Step 2: Solve for the atomic radius (r)
We are given the edge length (a = 310.2 pm). We can plug this value into the formula and solve for r:
310.2 pm = √2 * 4r
Now, we need to isolate r by dividing both sides of the equation by 4√2:
r = (310.2 pm) / (4√2)
r ≈ 109.8 pm
Therefore, the atomic radius of Floridium atoms in the FCC unit cell is approximately 109.8 pm to one decimal place.
learn more about atoms here
https://brainly.com/question/14352690
#SPJ11
A solution has [H3O+] = 2.8x10-5 M. Use the ion product constant of water K. = [H30 - ) [OH-] to find the [OH-] of the solution. Express your answer to two significant figures.
Answers
Answer:
Approximately \(3.6 \times 10^{-10}\; {\rm M}\), assuming that the solution is dilute and is at room temperature (such that \(K_\text{w} = 10^{-14}\).)
Explanation:
Look up the ion product constant of water:
\(K_{\text{w}} \approx 10^{-14}\).
In a dilute solution where water is the solvent, the product of \([{\rm {H_{3}O}^{+}}]\) and \([{\rm {OH}^{-}}]\) (concentration of \(\rm {H_{3}O}^{+}}\) ions and \({\rm {OH}^{-}}\) ions) is constantly equal to \(K_{\text{w}}\). (The unit of both \([{\rm {H_{3}O}^{+}}]\!\) and \([{\rm {OH}^{-}}]\!\) need to be \({\rm M}\).) In other words:
\([{\rm {H_{3}O}^{+}}]\, [{\rm {OH}^{-}}] = K_{\text{w}}\).
Rearrange this equation to find \([{\rm {OH}^{-}}]\) in terms of \(K_{\text{w}}\) and \([{\rm {H_{3}O}^{+}}]\):
\(\begin{aligned}[] [ {\rm OH^{-}} ] &= \frac{K_{\text{w}}}{[{\rm {H_{3}O}^{+}}]} \\ &= \frac{10^{-14}}{2.8 \times 10^{-5}} \\ &\approx 3.6 \times 10^{-10}\end{aligned}\).
Câu 1: Trình bày phương pháp hóa học nhận biết
a. 3 dung dịch: HCl , Na2SO4 , NaCl.
b. 3 dung dịch:NaOH , NA2CO3 , NaCl.
c. 4 dung dịch:HCl , H2SO4 , NaCl , Na2SO4.
Viết phương trình phản ứng xảy ra (nếu có)
Answers
Answer:
c
Explanation:
beacause Câu 1: Trình bày phương pháp hóa học nhận biết
Newton law of motion
Answers
Answer:
newtons law states that every body in the universe attracts every other body with a force which is directly proportional to the product of their masses and inversely proportional to square of the distance between their centers
Answer:
an object at rest tends to stay at rest
75% of the gases that make up the atmosphere are found below
16 kilometers
6 kilometers
10 kilometers
12 kilometers
Answers
salt a has a greater solubility in water than salt b. what can be said about their ksp values? g
Answers
If salt A has a greater solubility in water than salt B we can say that the Ksp value of salt A is greater than the Ksp value of salt B.
Solubility product constant (Ksp) is a measure of the solubility of an ionic compound in water. The Ksp value is dependent on the nature of the compound and the conditions under which it is dissolved. A higher Ksp value indicates that the compound is more soluble in water.
If salt A has a greater solubility in water than salt B, it means that salt A has a higher concentration of dissolved ions in water compared to salt B. This implies that the Ksp value of salt A is greater than the Ksp value of salt B, as a higher concentration of ions in solution requires a higher Ksp value to maintain equilibrium.
Therefore, we can conclude that the solubility of salt A is greater than salt B, and that the Ksp value of salt A is greater than the Ksp value of salt B.f f s
To know more about Ksp value, refer here:
https://brainly.com/question/12961072#
#SPJ11
5 - What is the pH of a solution with a
pOH of
8.6? What are the [+] and [OH']
concentrations?
Answers
Answer:
the pH solution is 4.9.
[\(H^{+}\)(aq)] = \(10^{-pH}\)
Explanation:
Suppose three different compounds composed of only nitrogen and oxygen were analyzed and the following data were obtained:
Mass of: Samples N O
Compound-A 1.500 g 0.955 g 0.545 g
Compound-B 2.000 g 0.933 g 1.067 g
Compound-C 3.000 g 0.913 g 2.087 g ———————————————————————————
a) Show that this data is consistent with the law of multiple proportions.
(b) If compound-B has the formula NO, what are the formulas of compounds A and C?
Answers
Answer:
I think it is b
Explanation:
but I'm not for sure that the answer is right
A student measured the gram weight of a
metal object to be 93.5g. According to the
supplier the object weighs 93.9g. What is the
error in the student's measurement?
Answers
Answer: 0.428%
Explanation: % Error = [(measured - Actual)/Actual]*100%
(93.9g - 93.5 g)/93.5g = 0.428%
Answer:
+0.4
Explanation:
For all those Acellus kids XD
how can offspring have traits that neither parent has?
Answers
When both parents shared different traits either it will be heterogeneous or homogenous traits,in that case offspring traits neither belongs to parents.However,chances are very that traits of children neither belongs to parents.
Unaffected parents can create impacted offsprings assuming that the two guardians are transporters (heterozygous) for the attribute being followed in the family. Latent traits are normally not communicated in each age. Finally, guys and females are similarly prone to communicate a latently acquired characteristic.
In the event that the latent characteristic is more than prevailing, the recessive traits will really become predominant and the predominant attribute will become recessive.Recessive alleles are meant by a lowercase letter (a versus A). Just people with an aa genotype will communicate a latent characteristic; consequently, posterity should get one passive allele from each parent to display a latent traits.
To know more about traits,visit here:
https://brainly.com/question/16307346
#SPJ4
why must we include units with a measurement?
Answers
Answer:
Units are used to show if a certain calculation is on track.
Explanation:
For example, 5.1 inches is way shorter than 5.9 inches.
i just need #2, its due any minute. giving extra points, and will mark brainliest!!!!!!!!
which unknown has the weakest attractive forces?
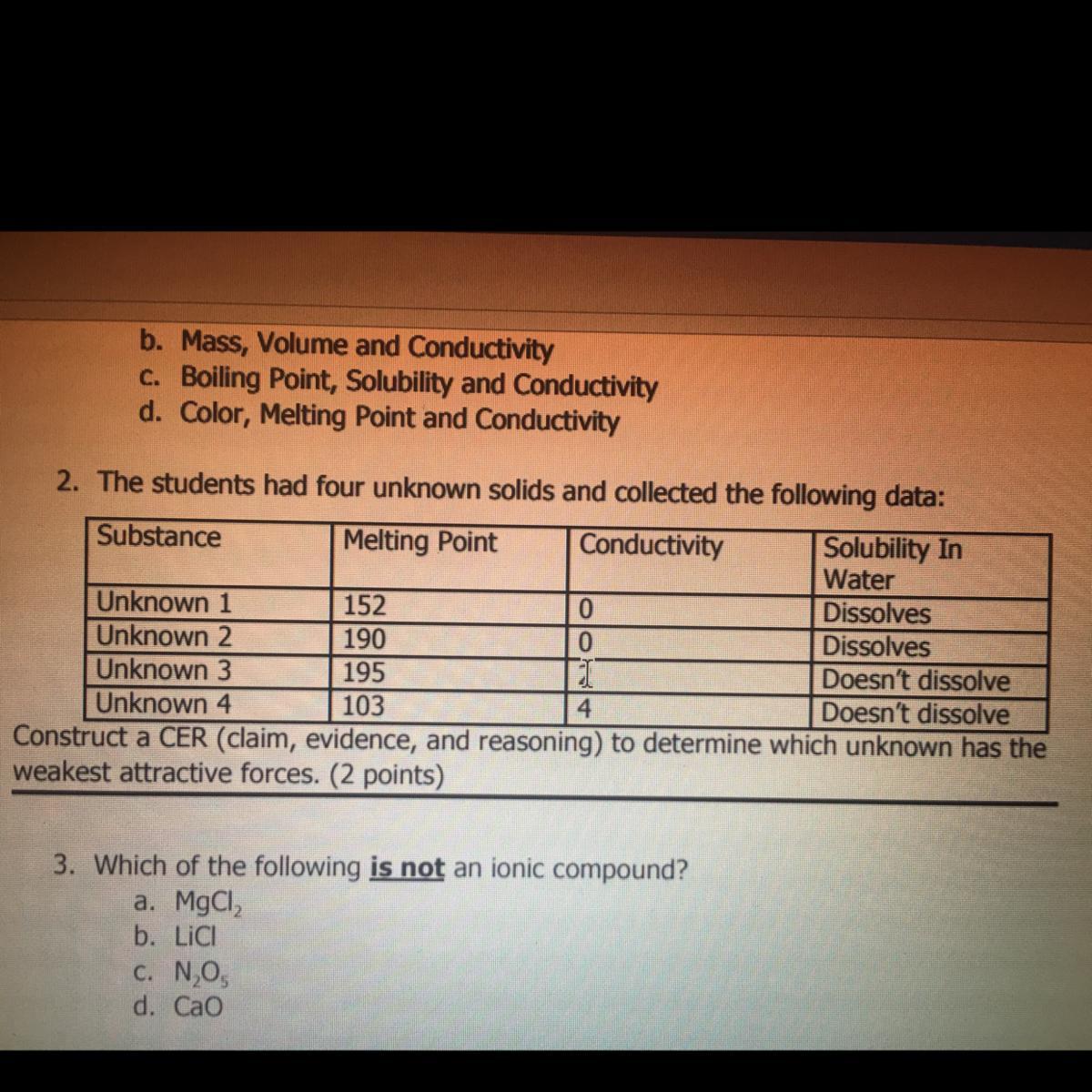
Answers
Answer:
Unknown 4
Explanation:
an object traviling around another object in space is in
Answers
Answer:
I think this the asnwer a satellit
Sorry if it incorrect
Have a nice day
Answer:
The Answer is Satellite.
Explanation:
An orbit is a regular, repeating path that one object in space takes around another one. An object in an orbit is called a satellite. A satellite can be natural, like Earth or the moon.
How many moles of O2 are used to produce 4 moles of NO?
Answers
The number of moles of O₂ used to produce 4 moles of NO is 2 moles
How do I determine the mole of O₂ used?First, shall write the balanced equation. This is given below:
N₂ + O₂ -> 2NO
From the balanced equation above,
2 moles of NO were obtained from 1 mole of O₂
With the above information, we can determine the number of moles of O₂ used to produce 4 moles of NO. This can be obtained as follow:
From the balanced equation above,
2 moles of NO were obtained from 1 mole of O₂
Therefore,
4 moles of NO will be obtain from = (4 × 1) / 2 = 2 moles of O₂
Thus, number of mole of O₂ used is 2 moles
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
!!!9POINTS!!!!! Based on the activity series, which of the reactions will occur?

Answers
The reactions that will occur for each activity series include:
A. Mg + NaNO₃ → will occur since Mg is more reactive than Na.B. AI+NISO₄→ will not occur since aluminum is less reactive than nickel.C. Zn + NaNO₃ - will occur since zinc is more reactive than sodium.D. Sn+ Zn(NO₃)₂ → will not occur since tin is less reactive than zinc.What are reactive metals?Reactive metals are metals that easily undergo chemical reactions with other substances, particularly with acids and water, to form new compounds. These metals are usually found in the lower part of the activity series, which means they have a high tendency to lose electrons and form cations.
Examples of reactive metals include alkali metals (such as lithium, sodium, and potassium) and alkaline earth metals (such as calcium and magnesium).
Find out more on reactive metals here: https://brainly.com/question/25103661
#SPJ1
Image trancribed:
Based on the activity series, which of the reactions will occur?
Least Reactive
Most Reactive Li Na K Mg Al Zn Fe Ni Sn Pb H Cu Ag Pt F₂ Cl₂ Br₂ I₂
Hint: Is the metal element more reactive than the metal ion in the compound?
A. Mg + NaNO3 →
B. AI+NISO4→
C. Zn + NaNO3 -
D. Sn+ Zn(NO3)2 →
Answer:A. Mg + NaNO₃ →
Explanation:
will occur since Mg is more reactive than Na.
Lead crystallizes in the face-centered cubic lattice. What is the coordination number for pb?.
Answers
The coordination number for pb will be 12
Four atoms make up each unit cell of the face-centered cubic (fcc), which has a coordination number of 12. Two atoms make up each unit cell of the body-centered cubic (bcc), which has a coordination number of eight.
What is Coordination number ?The number of atoms, ions, or molecules that a central atom or ion in a complex, coordination compound, or crystal holds as its closest neighbours.
The number of nearby atoms or ions that surround an atom or ion is known as the coordination number. The coordination number for FCC and HCP systems is 12.Tetracarbonylnickel, Ni(CO)4, for instance, has a coordination number of 4 due to the four CO ligands that are bonded to the Ni atom.Learn more about Coordination number here:
https://brainly.com/question/27289242
#SPJ4
Modeling Energy Changes
Student Guide
Answer in a copy and paste format, or using photos of what was said please.
Anyone who can do this will receive brainliest ofc!





Answers
In terms of the energy change in the reaction, the negative value indicates that the reaction is exothermic as the reaction releases 1560.74 kJ of energy for every mole of C2H6 that reacts with 7/2 moles of O2.
What is a model of chemical energy changes?The model of chemical energy changes is given below:
Balanced chemical equation:
C2H6 + 7/2 O2 → 2CO2 + 3H2O
Now, to calculate the energy change in the reaction, we will use a table of enthalpy values. The enthalpy change for each of the reactants and products is given in the table below:
Reactants:
C2H6: -84.68 kJ/mol
O2: 0 kJ/mol
Products:
CO2: -393.51 kJ/mol
H2O: -285.83 kJ/mol
The energy change in the reaction can be calculated using the formula:
ΔH = ∑(products) - ∑(reactants)
ΔH = [2(-393.51 kJ/mol) + 3(-285.83 kJ/mol)] - [-84.68 kJ/mol + 7/2(0 kJ/mol)]
ΔH = -1560.74 kJ/mol
Therefore, the energy change in the reaction is -1560.74 kJ/mol.
To create a model of the energy change in the reaction, we can use an energy level diagram. In this diagram, the energy of the reactants is shown on the left, the energy of the products is shown on the right, and the activation energy is shown as a barrier between them.
The energy level diagram for this reaction is shown below:
Reactants (C2H6 + 7/2 O2)
|
|
Activation energy
|
|
Products (2CO2 + 3H2O)
As shown in the diagram, the reactants have a higher energy level than the products, and the activation energy is required to get the reaction started.
Learn more about chemical energy changes at: https://brainly.com/question/26668994
#SPJ1
Complete them with correct formulas
Then balance them

Answers
Answer:
1. 2Ca + N\(_{2}\) → 2CaN
2. 4Li + O\(_{2}\) → 2Li\(_{2}\)O
3. 2KCl + BaF\(_2\) → 2KF + BaCl\(_2\)
4. CH\(_4\) + 2O\(_2\) → CO\(_2\) + 2H\(_2\)O
Be sure to balance the number of atoms on both sides of each equation only by adding coefficients to the compounds!!!! Those without a coefficient are meant to have a coefficient of 1.
PLEASE ANSWER QUICK IT'S URGENT 40 POINTS!!!!!
What type of attractive force is the arrow pointing at in the molecule?

Answers
Answer: C intermolecular force
Explanation:
The type of attractive force shown in the figure is intermolecular force. Option C is correct.
There are nonbonding forces of attraction between one individual molecule and another. These forces are referred to as intermolecular forces and are responsible for the physical behavior of the phases of matter, such as their ability to form solids, liquids, and gases.
The strength of the intermolecular forces varies depending on the type of substance and its molecular structure. For example, substances with strong intermolecular forces, such as water, have a higher boiling point and are more likely to exist as liquids or solids at room temperature, while substances with weak intermolecular forces, such as methane, have a lower boiling point and are more likely to exist as gases.
To know more about intermolecular force here
https://brainly.com/question/32203220
#SPJ2
The enthalpy of combustion for glucose c6h12o6(s) is -2801 kj/mol. a) write a balanced chemical reaction for the combustion of glucose.
Answers
The balanced chemical reaction for the combustion of glucose (C6H12O6) can be represented as follows:
C6H12O6(s) + 6O2(g) -> 6CO2(g) + 6H2O(g)
In this reaction, glucose (C6H12O6) reacts with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O).
To balance the equation, we need to ensure that the number of atoms of each element is the same on both sides of the equation. In this case, we have 6 carbon atoms, 12 hydrogen atoms, and 18 oxygen atoms on the left side (reactants). On the right side (products), we have 6 carbon atoms, 12 hydrogen atoms, and 18 oxygen atoms as well.
To balance the carbons, we place a coefficient of 6 in front of CO2. This gives us 6 carbon atoms on both sides. To balance the hydrogens, we place a coefficient of 6 in front of H2O. This gives us 12 hydrogen atoms on both sides. Finally, to balance the oxygens, we place a coefficient of 6 in front of O2. This gives us 18 oxygen atoms on both sides.
By balancing the equation, we ensure that the law of conservation of mass is obeyed. This means that the total number of atoms of each element remains the same before and after the reaction.
In summary, the balanced chemical equation for the combustion of glucose is:
C6H12O6(s) + 6O2(g) -> 6CO2(g) + 6H2O(g)
This equation shows that when glucose is combusted, it reacts with oxygen to produce carbon dioxide and water.
Learn more about combustion of glucose here:-
https://brainly.com/question/28980317
#SPJ11
what is the physical change of a liquid to a solid by the removal of heat?