7
Select the correct answer.
Which quantity is a graduated cylinder designed for measuring?
O A. length
ОВ.
mass
O C. temperature
D.
time
O E. volume
Answers
Related Questions
When alkaline hydrolysis was first invented what jobs were people hiring to do?
Answers
When alkaline hydrolysis was first invented, people were hired for various roles related to the process and implementation of this technology. Some of the jobs that emerged include Chemical engineers, Technicians and operators, Waste management specialists, Scientists and researchers.
Chemical engineers: These professionals played a crucial role in developing and optimizing the alkaline hydrolysis process. They were responsible for designing the equipment, developing the necessary chemical reactions, and ensuring the efficient operation of the system.
Technicians and operators: Skilled technicians and operators were hired to operate and maintain the alkaline hydrolysis equipment. They were trained to monitor the process parameters, handle the chemicals involved, and ensure the proper functioning of the system.
Waste management specialists: With the introduction of alkaline hydrolysis as a method for disposal of organic waste, specialized professionals in waste management were employed to oversee the proper handling and treatment of the waste materials. They were responsible for implementing safety protocols, managing waste streams, and complying with environmental regulations.
Scientists and researchers: Alkaline hydrolysis required scientific expertise for continuous improvement and innovation. Scientists and researchers were hired to study the process, analyze the results, and explore potential applications in various fields such as biofuel production and chemical synthesis.
Overall, the introduction of alkaline hydrolysis created employment opportunities for professionals in engineering, chemistry, waste management, and research, among others, as this technology gained recognition and adoption.
To know more about hydrolysis , click here, https://brainly.com/question/31132313
#SPJ11
A sample of gas has a volume of 2.00L at 25.0 degrees Celcius and 1.08atm. What volume ( in liters) will it have at 100 degrees Celcius and 1.5atm?
Answers
Answer:
Explanation:
The question requires us to calculate the volume (in L) of a gas under the conditions given.
The following information was provided by the question:
Initial volume of gas = V1 = 2.00 L
Initial temperature of gas = T1 = 25.0 °C
Initial pressure of gas = P1 = 1.08 atm
Final temperature of gas = T2 = 100 °C
Final pressure of gas = P2 = 1.50 atm
To solve this problem, we'll need to apply the equation of ideal gases (shown below) twice: first, to determine the number of moles of gas, and again to calculate the volume of gas under the final conditions. Note that we'll determine the number of moles of gas under the initial conditions because this amount won't change (as we're talking about the same sample of gas).
\(undefined\)label the following graph, which indicates the temperature versus the percent of dna that is single stranded.
Answers
This graph shows the relationship between temperature and the percentage of DNA that is single-stranded. As the temperature increases, the percentage of single-stranded DNA decreases. At temperatures below 70°C, the majority of DNA is single-stranded, but at higher temperatures, double-stranded DNA becomes more prevalent.
It is important to note that DNA is a double-stranded molecule and its stability is affected by temperature. As the temperature increases, the hydrogen bonds that hold the two strands of DNA together begin to break, causing the DNA to become single-stranded. This process is called DNA melting or denaturation.
The percent of DNA that is single-stranded at a given temperature can be measured and plotted on a graph, with the temperature on the x-axis and the percent of single-stranded DNA on the y-axis. This type of graph can be used to study the stability of different DNA molecules and to compare the melting temperatures of different DNA sequences.
See more about single-stranded in:
https://brainly.com/question/30545647
#SPJ11
how can i find wavelength in a wave?
Answers
Wavelength (L) is calculated using: L = gT²/2π, here g=9.8 m/s2 and T is wave period in seconds.
What is wavelength?Wavelength of a wave describes how long the wave is and the distance from the "crest" (top) of one wave to the crest of next wave is called wavelength. We can also measure from the "trough" (bottom) of one wave to trough of next wave and get the same value for the wavelength.
We measure wavelength in following ways:
Use photometer to measure the energy of wave.
Convert energy into joules (J).
Divide energy by Planck's constant, 6.626 x 10⁻³⁴, to get the frequency of wave.
Divide speed of light, ~300,000,000 m/s, by frequency to get wavelength.
To know more about wavelength, refer
https://brainly.com/question/10750459
#SPJ9
3. Which two atoms could potentially form alloys?
Tin and Copper
Bromine and Potassium
Sodium and Chloride
Hydrogen and Oxygen
Answers
Answer:
Tin and Copper
Explanation:
The two atoms that could potentially form alloys are the tin and copper atoms.
Alloys are mixtures formed between two or more metals.
The goal of forming alloys is to take properties of one metal and add to that of another metal so as to enhance both materials.
Alloys are only formed between metals.
The only metal pair given from the choices is that of tin and copper.
Therefore, the two atoms that could potentially form alloys is tin and copper.
at what points does the object accelerate please explain
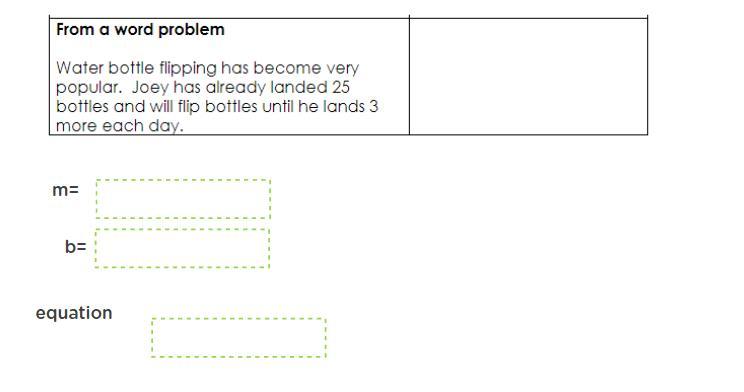
Answers
Answer:
M=3
B=25
Equation= 3x+25
Explanation:
M is your slope and if you follow the slope- intercept formula (y=m+b) you just need to plug in the numbers.
Thus your answer:
M=3
B=25
Equation= 3x+25
Your family is planning a trip to South America for Winter Break. Describe what type of clothes you
would pack in your suitcase. Justify your response using your knowledge of the seasons.
Answers
Answer:
okey if I'm going on south America for the winter break i would basically pack baggy clothes ,sweater,jackets,wollen hats,wollen clothes,socks,shoes,scarf,heavy and wollen one thats all ig
2. Which of the following does not have a nucleus? a. prokaryotes b. eukaryotes c. prokaryotes and eukaryotes
Answers
Answer:
Erythrocytes
Erythrocytes do not contain a nucleus. This would make answer choice "A" correct. Erythrocytes are red blood cells and these cells differ from the other cells of the body because of the absence of the nucleus. All the other cells noted have nuclei.
Explanation:
Have A Wonderful Day !!
Answer:a. Prokaryotes
Explanation:
If an iv is mixed so that each 150 ml contains 440. mg of the drug lidocaine, how many minutes will it take for 650 mg of lidocaine to be administered if the rate is set at 4.0 ml/min?
Answers
It will take approximately 16.25 minutes for 650 mg of lidocaine to be administered at a rate of 4.0 ml/min. To find the time it takes to administer 650 mg of lidocaine, we need to use the given information.
Each 150 ml of IV contains 440 mg of lidocaine. Therefore, in 1 ml of IV, there are (440 mg / 150 ml) = 2.93 mg of lidocaine. Since the rate is set at 4.0 ml/min, the amount of lidocaine administered per minute would be (4.0 ml/min * 2.93 mg/ml) = 11.72 mg/min. To calculate the time it takes to administer 650 mg of lidocaine, we divide the desired amount (650 mg) by the rate (11.72 mg/min): 650 mg / 11.72 mg/min ≈ 55.50 min.
Therefore, it will take approximately 16.25 minutes for 650 mg of lidocaine to be administered at a rate of 4.0 ml/min.
The time it takes to administer 650 mg of lidocaine can be found using the given information. Each 150 ml of IV contains 440 mg of lidocaine, so in 1 ml of IV, there are 2.93 mg of lidocaine. The rate at which the IV is set is 4.0 ml/min. To find the amount of lidocaine administered per minute, we multiply the rate by the amount of lidocaine per ml, which equals 11.72 mg/min. To calculate the time it takes to administer 650 mg of lidocaine, we divide the desired amount by the rate, which gives us 55.50 min. Therefore, it will take approximately 16.25 minutes for 650 mg of lidocaine to be administered at a rate of 4.0 ml/min.
To know more about lidocaine visit:
https://brainly.com/question/33462292
#SPJ11
Add labels for mass extinction and adaptive radiation:

Answers
Answer:
Couldn't figure out how to edit the other one to attach the image, but here is it with the labels. Also put the same explanation down below so you don't have to go to the other if you wanted to look at it in conjunction with the image.
Explanation:
Mass extinctions are periods of time where the organisms are essentially wiped out in an extremely brief period of time. You can see how the species just drops off. Adaptive radiation occurs when there is a diversification of a group of organisms as evident by the rise in the number of marine animal groups shown by the bracketed area.

"Benzene is an inflammable liquid" is it a chemical property of Benzene
Answers
Answer:
df
Explanation:
fawfe
Answer:
yes it is
hope it helps you
an
a transition metal with 91 protons and electrons?
Answers
Hshsbsbwhwhhsnsnanananananababsbsbsbsbsbsbababjaajajajnsnsnsnana
Answer:
Protactinium
Explanation:
the atomic number lines up with the number of protons and electrons an element has.
Given the pKa’s for H2CO3: pKa1 = 6.35; pKa2=10.33, what is the pKb1 of CO32- (Kb1 is the equilibrium constant of the reaction: CO32- + H2O ⇌ HCO3- + OH-)?
(A) 14.00
(B) 10.33
(C) 3.67
Answers
To determine the pKb1 of CO32-, we can use the relationship between pKa and pKb for conjugate acid-base pairs:
pKa + pKb = pKw
where pKw is the ionization constant of water, which is approximately 14. Therefore, we can rearrange the equation to solve for pKb:
The pKb value represents the negative logarithm of the equilibrium constant (Kb) for the reaction of a base with water. In this case, we are interested in the equilibrium reaction between CO32- and water, which can be represented as CO32- + H2O ⇌ HCO3- + OH-.
By utilizing the relationship pKa + pKb = pKw, we can rearrange the equation to solve for pKb. Given that pKa1 of H2CO3 is 6.35, we subtract this value from pKw (approximately 14) to obtain pKb1
pKb = pKw - pKa
pKb1 = 14 - 6.35 = 7.65
Since none of the given answer choices matches the calculated value, it seems there might be an error or omission in the available options. Please double-check the answer choices provided or refer to additional information to obtain the correct pKb1 value for CO32-.
Learn more about equilibrium constant: brainly.com/question/29809185
#SPJ11
Use the exponential notation to express the physical quantities
a. V= 15. 50 ml to L
b. M= 5. 00mg to g
c. M= 2000 to g
d. Λ= 256. 4nm to m
Answers
b. M = 0.00500 g (or 5.00 x 10^-3 g)
c. M = 2.000 kg (or 2.000 x 10^3 g)
d. Λ = 2.564 x 10^-7 m (or 2.564 nm)
Therefore, V = 1.550 x 10^-2 L (expressed in exponential notation)
b. M = 5.00 mg = 0.00500 g (since there are 1000 mg in 1 g)
Therefore, M = 5.00 x 10^-3 g (expressed in exponential notation)
c. M = 2000 g
Therefore, M = 2.00 x 10^3 g (expressed in exponential notation)
d. Λ = 256.4 nm
Since there are 10^9 nm in 1 m, we can convert Λ to meters as follows:
Λ = 256.4 x 10^-9 m
Therefore, Λ = 2.564 x 10^-7 m (expressed in exponential notation)
If you place a piece of iron at 90 °C into water that is 25 °C, in which direction heat will flow?
Answers
Answer:
Determine the final temperature when a 25.0 g piece of iron at 85.0 °C is placed into 75.0 grams of water at 20.0 °C.
First some discussion, then the solution. Forgive me if the points seem obvious:
a) The colder water will warm up (heat energy "flows" in to it). The warmer metal will cool down (heat energy "flows" out of it).
b) The whole mixture will wind up at the SAME temperature. This is very, very important.
c) The energy which "flowed" out (of the warmer water) equals the energy which "flowed" in (to the colder water)
Explanation:
Which characteristic will the animal in the image pass on to its offspring
Help me out please

Answers
Answer:
Sea turtle
Is the animal in the image
11. An alloy contains 62 % by mass of aluminum and 38% by mass of unknown element .If 10.0
grams of this alloy has a volume 4.20 cm³ ,use the table of density below to identify the
unknown element in the alloy.
Element
Density g/cm³
(A) Beryllium
Copper
8.96
Aluminum
2.70
(B) Copper
Beryllium
1.85
(C) Iron
Iron
7.87
(D) Silver
Silver
10.49
Answers
Based on the given information and the densities provided in the table, the unknown element in the alloy is most likely Beryllium. option(a)
To identify the unknown element in the alloy, we need to compare the density of the alloy with the densities of the elements listed in the table.
The density of the alloy can be calculated using the given information. We know that 10.0 grams of the alloy has a volume of 4.20 cm³. Density is defined as mass divided by volume, so we can calculate the density of the alloy as:
Density = Mass / Volume = 10.0 g / 4.20 cm³ ≈ 2.38 g/cm³
Now, we compare the calculated density of the alloy (2.38 g/cm³) with the densities listed in the table. From the given options, the closest density is that of aluminum, which is 2.70 g/cm³. The alloy's density is lower than the density of aluminum, which means it must contain an element with a lower density than aluminum.
The unknown element in the alloy is most likely Beryllium (option A) with a density of 1.85 g/cm³. The combination of 62% aluminum and 38% beryllium in the alloy would result in a density close to the calculated value of 2.38 g/cm³. option(a)
For such more questions on densities
https://brainly.com/question/1749900
#SPJ8
what is 1006 in scientific notation?
Answers
Answer:
1.006 * 103
Explanation:
Add the number between 1 and 9 and add a decimal accordingly . so the answer is 1.006 multiplied by 10 raised to power 3
give me the type of reactions ___ CuCO3 → ___ CuO + ___ CO2
Answers
When green copper carbonate{CuCO3} is heated it decomposes forming copper oxide {CuO} and carbon dioxide {CO2}. This is a decomposition reaction.
gallium has two naturally occurring isotopes, and . if has an atomic mass of 68.926 amu, has an atomic mass of 70.925 amu, and the atomic mass of gallium is 69.723 amu, what is the percent abundance of ?
Answers
If has an atomic mass of 68.926 amu, has an atomic mass of 70.925 amu, and the atomic mass of gallium is 69.723 amu, the percent abundance of 70Ga is 101.68%.
To find the percent abundance of an isotope, you need to determine its relative abundance compared to the total amount of the isotopes present. Here's how you can do it for the isotope 70Ga:
Calculate the fraction of 70Ga in the sample:
fraction of 70Ga = (mass of 70Ga) / (atomic mass of Gallium)
fraction of 70Ga = (70.925 amu) / (69.723 amu)
fraction of 70Ga = 1.0168
Convert the fraction to a percentage:
percentage of 70Ga = (fraction of 70Ga) * 100
percentage of 70Ga = (1.0168) * 100
percentage of 70Ga = 101.68%
To know more about isotope
https://brainly.com/question/11680817
#SPJ4
1. The compound 2NaCl consists of A.2 atoms of sodium element B. 1 atom of
chlorine element C. 3 atoms of sodium element D. 1 atom of sodium element
2. The valency of iron in iron (III) sulphate is A.3 B.2
C.4
D.5
3.The systematic name of the compound Cuso, is A. Copper (I) sulphate B.Copper
(II) tetraoxosulphate C.Copper (II) tetraoxosulphate (IV) D.Copper (II)
tetraoxosulphate (VI)
Answers
Answer:
1. The compound 2NaCl consists of:
» B. 1 atom of chlorine element.
» D. 1 atom of sodium element.
2. The valency of iron in iron (III) sulphate is:
» A. 3 [ valency of only iron ]
» B. 2 [ valency of only sulphate ion ]
3.The systematic name of the compound CuSO4:
» B. Copper (II) sulphate
Explanation:
\(.\)
Answer:
1) A.
2) A.
3) A.
if 1.0230 gram of norborneol were isolated from a hydration reaction of 1.245 grams of norbornene, what is the percent yield?
Answers
The percent yield of norborneol is approximately 82.2%.
To calculate the percent yield, we use the formula: percent yield = (actual yield / theoretical yield) * 100%.
First, we need to determine the theoretical yield, which is the amount of norborneol that would be produced if the reaction proceeded with 100% efficiency.
The balanced equation for the hydration reaction of norbornene to norborneol is not provided, so we cannot determine the stoichiometry of the reaction.
Assuming the reaction proceeds according to a 1:1 stoichiometry, the theoretical yield of norborneol would be equal to the amount of norbornene used in the reaction (1.245 grams).
Now, we can calculate the percent yield using the actual yield (1.0230 grams) and the theoretical yield (1.245 grams): percent yield = (1.0230 / 1.245) * 100% ≈ 82.2%.
Therefore, the percent yield of norborneol from the hydration reaction is approximately 82.2%.
To know more about "Stoichiometry" refer here:
https://brainly.com/question/28780091#
#SPJ11
What is the molarity of a solution in which 55.49 g of calcium chloride is dissolved in enough water to make 500. ml of solution?
Answers
The molarity of a solution in which 55.49 g of calcium chloride is dissolved in enough water to make 500. ml of solution will be 1M.
The quantity of a substance in a specific volume of solution is known as its molarity (M). The number of moles of a solute per liter of a solution is known as molarity. The term "molarity" can also refer to a solution's molar concentration.
Calculation of molarity is shown as:
Given data:
Mass of \(CaCl_{2}\) =55.49g
Molar mass of \(CaCl_{2}\) =40+35+35=110g
Moles = mass / molar mass
Moles = 55.43 / 110
Moles = 0.50 mol
Molarity can be calculated by using the formula:
Molarity = moles of solute / solution
Molarity = 0.5 × 1000 / 500
Molarity = 1 M
Therefore, the molarity of the calcium chloride solution will be 1 M.
To know more about molarity
https://brainly.com/question/8732513
#SPJ4
3NO₂ + H₂O → 2HNO3 + NO 3NO₂ + H₂O → 2HNO3 + NO
How many moles of nitric acid are produced from 300 mol of nitrogen dioxide?
Answers
Answer:
Ken is planting vegetables in his garden. He has 20 seeds. Determine whether 20 is prime or composite number. If it is composite, list all of the ways Ken can arrange the seeds in even rows.
A pure form of matter is isolated and tested. no physical procedures can produce simpler forms of matter but heating the sample produces a liquid and a gas. the sample of matter must be a(n)?
Answers
The sample of matter that has been isolated and tested, and can only be broken down into a liquid and a gas by heating it, must be a compound.
A synthetic compound is a substance made out of numerous indistinguishable particles containing molecules from more than one compound component kept intact by substance bonds. A particle comprising of iotas of only one component is subsequently not a compound.
A pure form of matter that cannot be further broken down into simpler forms by physical procedures is called an element. A compound is a substance that is composed of two or more different elements chemically combined. In this scenario, heating the sample of matter resulted in two different forms: a liquid and a gas.
This suggests that the sample was composed of two or more substances that were chemically combined. Therefore, the sample of matter is a compound.
Know more about compound:
https://brainly.com/question/14117795
#SPJ11
4. C2 JUN 09 Q7c
Propene reacts with hydrogen bromide to give 2-bromopropane as the major product.
(1) Using the reaction scheme below, show the mechanism of the reaction using curly
arrows and full negative and positive charges as appropriate.
(2)
H,C
H
c=c
H
H
H-Br
CH, H
H-C-C-H
Br H
CH, H
1 1
H-C-C-H
Br H
2-bromopropane
(ii) State briefly, why 2-bromopropane, rather than 1-bromopropane, is the main product
of this reaction.
[1]
Answers
Explanation:
(1) The mechanism of the reaction between propene and hydrogen bromide to give 2-bromopropane is as follows:
Protonation of the alkene: A proton from hydrogen bromide (HBr) attacks the alkene, forming a carbocation intermediate.
Bromine addition: Bromine (Br) adds to the carbocation intermediate to form an intermediate bromonium ion.
Deprotonation: A proton from the bromonium ion is removed by a water molecule or another molecule, producing the final product 2-bromopropane.
The mechanism can be represented using curly arrows as follows:
H,C
H
c=c
H
H
H-Br
CH, H
H-C-C-H
Br H
CH, H
1 1
H-C-C-H
Br H
2-bromopropane
(2) 2-bromopropane is the main product of this reaction because of the stereochemistry of the reaction. When the carbocation intermediate forms, the bromine atom has a preference for adding to the face of the alkene that has the least number of hydrogen atoms. This leads to the formation of the 2-bromopropane, which has the bromine atom attached to the carbon atom with two hydrogen atoms. On the other hand, the formation of 1-bromopropane, which has the bromine atom attached to the carbon atom with three hydrogen atoms, is less favorable. This is why 2-bromopropane is the main product of this reaction.
The pattern of atoms and molecules give matter it’s ______
What is the blank?
Answers
Answer: Try mass that’s the best guess I can give off the top of my head
Explanation: An atom is the smallest unit of matter that retains all of the chemical properties of an element. Atoms come together which forms molecules. These molecules interact to form solids, gases, or liquids.
A water sample that has been diluted to 10-3 has been diluted by a factor of _______ times.
a. 1/300
b. 300
c. 1/1,000
d. 1,000
e. 3,000
Answers
In this case, a water sample has been diluted to 10^-3, which means it has been diluted by a factor of 1,000 times. So, the correct answer is:
The answer is option b. 300.
When a solution is diluted, it means that more solvent (usually water) has been added to decrease the concentration of the solute. The dilution factor is the ratio of the final volume of the solution to the initial volume of the solution.
In this case, the water sample has been diluted to 10-3, which means that the concentration of the original solution has been reduced by a factor of 10-3.
To calculate the dilution factor, we need to take the reciprocal of the concentration reduction factor:
Dilution factor = 1/10-3 = 1,000
So the water sample has been diluted by a factor of 1,000 times.
Therefore, the correct answer is option b. 300 is not the dilution factor, but rather the concentration reduction factor (1/300), which is the reciprocal of the dilution factor.
Learn more about :
dilution factor : brainly.com/question/30893079
#SPJ11
Predict the missing component
in the nuclear equation.
230 Th
90
→>>
226 Ra + X
88
Answers
The missing component of the nuclear equation is 4 2He
Data obtained from the questionThe following data were obtained from the question:
Equation: 230 90Th --> 226 88Ra + XValue of X =?How to determine the missing component, XThe missing component, X of the nuclear equation can be obtained as illustrated below:
230 90Th --> 226 88Ra + a bX
230 = 226 + a
Collect like terms
a = 230 - 226
a = 4
90 = 88 + c
Collect like terms
c = 90 - 88
c = 2
a cX => 4 2X => 4 2He
Thus, the complete equation is:
230 90Th --> 226 88Ra + 4 2He
Therefore, the missing component, of the equation is 4 2He
Learn more about nuclear equation:
https://brainly.com/question/19752321
#SPJ1
Calculate the grams of CaCl2 necessary to make a 0.15Msolution.
Answers
Answer: mass m = M·c·V
Explanation: M(CaCl2) = 110.98 g/mol, c= 0.15 mol/l,
n=m/M= cV, volume of Solution is not mentioned