Answers
The concentrations of NO2(g) and N2O4(g) at equilibrium is 0.79 M.
What is equilibrium?Equilibrium is defined as the situation where market supply and demand are in balance and prices are constant. It is possible to utilize a mathematical formula to determine the equilibrium price. The demand and supply quantities form the basis of the equilibrium pricing formula.
Given
Temperature = 500K
Moles = 2.57 mole
Volume = 3.25 L
Concentration = Moles / Volume
Concentration = 2.57 / 3.25
Concentration = 0.79 M
Thus, the concentrations of NO2(g) and N2O4(g) at equilibrium is 0.79 M.
To learn more about equilibrium, refer to the link below:
https://brainly.com/question/28527601
#SPJ1
Related Questions
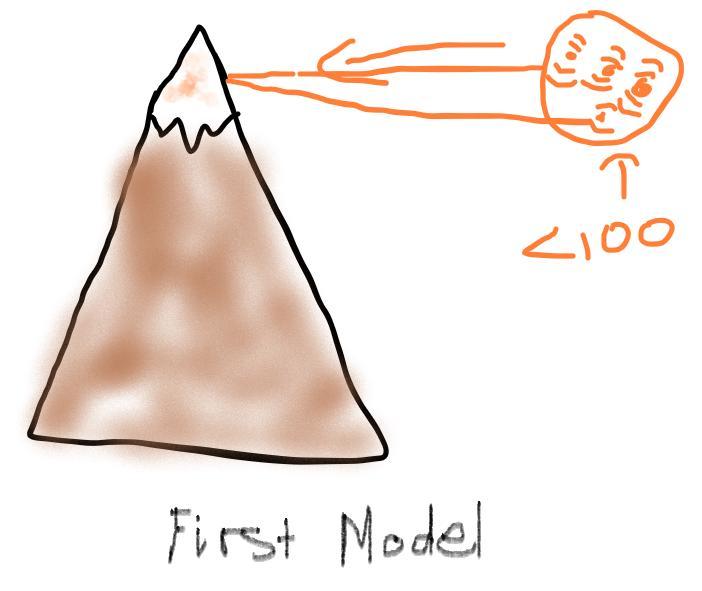
Air pressure decreases as elevation increases. Identify how the boiling point of water on top of a mountain would be different from its boiling point at sea level. Draw two models to show how the particle motion and the state of water on the mountain top and at sea level would change if you kept adding thermal energy to water that was already at 100°C. Label your models to show what happens to the temperature as the energy is added.
Answers
Answer:
The Boiling Point would be below a hundred degrees on a mountain than at sea level.
Explanation:
The Boiling Point is below a hundred degrees, because the air pressure at high altitudes is very low. ( -- < 100)
The Boiling Point for at sea-level would-be a hundred degrees because the air pressure is very high at lower altitudes.
First Model: is shown below, and it shows that the motion of thermal energy particles are moving fast when below 100 degrees ( -- < 100).
Second Model: is shown below, and it shows that the motion of thermal energy are moving fast when at 100 degrees (--- = 100)
Hope this helps!

What is the definition of lava?
Answers
Answer:
Lava, magma (molten rock) emerging as a liquid onto Earth's surface. The term lava is also used for the solidified rock formed by the cooling of a molten lava flow.
Explanation:
Suppose that you have 70 grams of calcium chloride (CaCl2) contaminated with 5% sodium chloride (NaCl) and 2% sand. You dissolve the impure solid in 54 ml of hot water, and allow it to cool and crystallize. What would be the maximum mass (grams) of purified CaCl2 that would you would be able to recover
Answers
Answer:
65.1 grams
Explanation:
The percentage of impurities in the contaminated CaCl₂ sample is:
% of NaCl + % of sand = 7%Meaning that the purity of the sample is (100 - 7) 93%.
Now we calculate the mass of pure CaCl₂ in the contaminated sample:
70 g * 93/100 = 65.1 gThus the answer is 65.1 grams, as that amount is how much CaCl₂ is in the sample and it would be impossible to obtain more than that.
how many moles are in 8.2 x 10^22molecules of N2I6?
Answers
Answer:
First, find out how many moles of N2I6 you have. Then convert that to grams.
molar mass N2I6 = 789 g
moles N2I6 = 8.2x1022 molecules N2I6 x 1 mole/6.02x1023 molecules = 1.36x10-1 moles = 0.136 moles
grams N2I6 = 0.136 moles x 789 g/mole = 107 g = 110 g (to 2 significant figures)
Which of the following definitions best describes the term "vapor pressure?a. Pressure and temperature values on a phase diagram where two phases of a substance coexist. b. In equilibrium with the liquid phase, the pressure exerted by a gas. c. Aspecific temperature and pressure at which the liquid and gas phases of a substance have the same density and are indistinguishable from each other. d. The temperature and pressure at which all three phases of a substance coexist. Under these conditions, freezing and melting, boiling and liquefaction, and sublimation and deposition all proceed at the same rate. e. The temperature at which the vapor pressure of a liquid equals 1 atm.
Answers
Answer:
b. In equilibrium with the liquid phase, the pressure exerted by a gas.
Explanation:
When a liquid is warmed up to a temperature , it starts vaporising . The liquid is turning into gas and gas is turning into liquid at different rates . Initially the rate of former is higher but gradually the difference of rate between them decreases to zero . At this point the rate of conversion of liquid into gas and rate of conversion of gas into liquid becomes equal . This is called dynamic equilibrium point .
If we change the temperature , the equilibrium gets disturbed .
At this point the pressure exerted by the gas is called the vapour pressure of the liquid .
So option b ) is correct .
At the equilibrium point, the pressure exerted by the gas molecules to the liquid molecules has been termed the Vapor pressure. Thus, statement b is correct.
The vaporization has been the process of conversion of liquid to the gaseous state with the rise in temperature. The liquids attaining a certain temperature have been vaporized into the gaseous state.
Initially, the gas phase has been less in concentration, thus the rate of formation of gas has been greater.
After a certain amount of time, the gas phase starts to cool down and converts to the liquid state. The rate of formation of the liquid has been slower.
The time when the rate of formation of liquid, and the rate of formation of gas has been equal is termed as the equilibrium point. At the equilibrium point, the pressure exerted by the gas molecules to the liquid molecules has been termed the Vapor pressure.
Thus, statement b is correct.
For more information about the Vapor pressure, refer to the link:
https://brainly.com/question/8646601
The student carries out a second experiment to investigate whether another substance, copper(II) oxide, is a better catalyst than manganese(IV) oxide. Describe how the second experiment is carried out. You should state clearly how you would make sure that the catalyst is the only variable.
Answers
The experiments showed that the cubic cuprites for Cu\(_2\)O and the face-centered cubic (fcc) crystal structure of copper (Cu) metal.
X-ray Diffraction (XRD), Energy Disper-sive X-ray Fluorescence (EDXRF), Scanning Electron Microscope (SEM), and Transmission Electron Microscope (TEM) were used to characterise the Cu/Cu\(_2\)O nanoparticles. The experiment showed that the cubic cuprites for Cu2O and the face-centered cubic (fcc) crystal structure of copper (Cu) metal. Hydrazine was used as the reducing agent and copper sulphate pentahydrate as the precursor in a modified chemical reduction process to create copper/copper oxide (Cu/Cu2O) nanoparticles in an aqueous media.
To know more about experiments, here:
https://brainly.com/question/1347425
#SPJ1
The weather balloon is inflated to a volume of 605dm3 at STP. The balloon is heated to 35 degree Celsius. What would be its volume at 75 cmHg?
Answers
The volume of the weather balloon at 75 cmHg is (n₁ * R * T₂) / P₂ = (n₁ * 0.0821 L·atm/(mol·K) * 308.15 K) / 0.9868 atm.
The volume of the weather balloon at 75 cmHg when it is heated to 35 degrees Celsius, we can use the ideal gas law, which states that the product of pressure (P) and volume (V) of a gas is proportional to the product of the number of moles (n) and the temperature (T) in Kelvin.
PV = nRT
Where R is the ideal gas constant.
First, let's convert the initial volume from dm³ to liters:
Initial volume = 605 dm³ = 605 L
At STP (Standard Temperature and Pressure), the temperature is 273.15 K and the pressure is 1 atmosphere (atm).
Using the initial conditions:
P₁ = 1 atm
V₁ = 605 L
T₁ = 273.15 K
We can calculate the number of moles (n₁) of gas using the ideal gas law:
n₁ = (P₁ * V₁) / (R * T₁)
Now, we need to find the final volume (V₂) at 75 cmHg and 35 degrees Celsius.
Converting 75 cmHg to atmospheres:
P₂ = 75 cmHg * (1 atm / 76 cmHg) ≈ 0.9868 atm
so,
P2 = 75 cmHg / 76 cmHg/atm = 0.9868 atm.
Now we can substitute the values into the equation and calculate V2:
V2 = (1 atm / 0.9868 atm) * (T2 / 308.15 K) * (605 liters).
Please provide the new temperature (T2) in Celsius, and I will calculate the volume for you.\((n₁ * R * T₂) / P₂ = (n₁ * 0.0821 L·atm/(mol·K) * 308.15 K) / 0.9868 atm\)
For more such questions on volume
https://brainly.com/question/27100414
#SPJ11
[H+] for a solution is
1.87 x 10-11 M.
This solution is:
A. Acidic
B. Basic
C. Neutral
Answers
Answer:
Basic
Explanation:
pH = -log[H⁺] = - log(1.87 x 10⁻¹¹) = 10.73 (basic solution)
Answer:
basic
Explanation:
How would you monitor the progress of a neutralization reaction? Question 2 options: We will use a funnel to separate the solid as it forms We will use a balance to see the changes in mass We will use a thermometer to check the changes in temperature We will use an acid-base indicator to see changes in color depending on the pH
Answers
Answer:
We will use an acid-base indicator to see changes in colour depending on the pH
Explanation:
The pH changes during a titration, so you could use an acid-base indicator to follow the changes in pH.
A is wrong. An acid-base titration does not usually form a solid, and it would be impractical to isolate a solid with a funnel.
B is wrong. There are no changes in mass.
C is wrong. Any changes in temperature would be too small to measure precisely with an ordinary thermometer.
The best way to monitor the progress of a neutralization reaction such as acid-base titration: D. Use an acid-base indicator to observe the changes in color depending on the pH.
The chemical reaction that occurs when you mix an acid and a base together is referred to as neutralization reaction.
In a neutralization reaction, what is formed is salt and water.
Acid-base titration is a neutralization method.
During acid-base titration, the neutralization reaction that occurs is usually monitored by observing the pH changes that occurs.
Change in pH is an indicator that there is progress in the neutralization reaction.
An acid-base indicator, can be used to detect the changes that occur via the pH changes in relation to the color change.
Therefore, the best way to monitor the progress of a neutralization reaction such as acid-base titration: D. Use an acid-base indicator to observe the changes in color depending on the pH.
Learn more about neutralization reaction here:
https://brainly.com/question/12442828
gas laws escape room
Answers
Answer:
what are you asking
Explanation:
The gas laws are Boyle's law, Charles law, ideal gas law, and Gay-Lussac law.
What are the different gas laws?The first law is Boyle's law that states that at constant temperature, the pressure varies with volume of the gas.
The second law is Charles's law that states that at constant pressure, the volume of the gas is directly proportional to the temperature of the gas.
Above, both laws form the ideal gas law. PV = nRT.
Thus, the gas laws are Boyle's law, Charles law, ideal gas law, and Gay-Lussac law.
Learn more about gas laws
https://brainly.com/question/12667831
#SPJ2
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
9. Which type of reaction is shown by the equation below?
PAO10(s) + 6 H20 (1) ► 4 H3PO4(ay)
Synthesis
Combustion
оооо
o
Double replacement
Decomposition
Answers
How can you reduce the amount of waste you send to landfills?
Answers
Answer:
for reducing your landfill contribution
Reduce and reuse: The most obvious and easiest way to reduce your landfill contribution is by reducing and reusing as much as possible. ...
1,Recycle: ...
2,Compost: ...
3,Electric or gas dryer: ...
4,Drive less: ...
5,Reusable water bottle: ...
6,Plant a tree: ...
7,Recycle electronics
There are several ways you can reduce the amount of waste you send to landfills:
Reduce your consumption: One of the most effective ways to reduce waste is to simply consume less. This can involve reducing your use of single-use plastics, choosing products with minimal packaging, and opting for products that are made to be reused or recycled.
Recycle: Recycling is a process that converts waste materials into new products. By recycling, you can help to conserve natural resources and reduce the amount of waste that goes to landfills.
Compost: Composting is the process of breaking down organic material, such as food waste and yard trimmings, into a soil-like substance called compost. This can be used to enrich the soil and help plants grow.
Donate or sell gently used items: Rather than throwing away gently used items, consider donating them to a thrift store or selling them online. This can help to extend the life of these items and reduce the amount of waste that ends up in landfills.
Reuse: Look for ways to reuse items rather than throw them away. For example, you can use a reusable water bottle instead of disposable plastic bottles, or bring a reusable bag to the grocery store instead of using plastic bags.
Learn more about Chemical landfills here: https://brainly.com/question/9085477
Does any know the answer to the first three question

Answers
1. C) Molarity is indirectly related to volume.
2. A) The CaCl2 beaker has more ions in solution.
3. the molarity of the NaCl solution is 0.0513 M.
How to find the molarityStep 1: Convert 30g of NaCl to moles.
The molar mass of NaCl is 58.44 g/mol. To convert 30 g to moles, divide by the molar mass:
30 g NaCl / 58.44 g/mol = 0.513 mol NaCl
Step 2: List Given and asking information.
Given:
Mass of NaCl = 30g
Volume of solution = 10.0L
Asking:
Molarity of the solution = ?
Step 3: set Molarity Formula and plug the mole/ Volume into the formula.
Molarity = moles of solute / volume of solution
Molarity = 0.513 mol / 10.0 L = 0.0513 M
Therefore, the molarity of the NaCl solution is 0.0513 M.
Learn more about molarity at
https://brainly.com/question/14469428
#SPJ1
It took 2.30 minutes using a current of 3.00 A to plate out all the copper from 0.300 L of a solution containing Cu2 . What was the original concentration of Cu2
Answers
Answer:
7.16 × 10⁻³ M
Explanation:
Let's consider the reduction reaction of copper during the electroplating.
Cu²⁺(aq) + 2 e⁻ ⇒ Cu(s)
We can calculate the moles of Cu²⁺ present in the solution using the following relations.
1 A = 1 C/s.1 min = 60 s.1 mole of electrons has a charge of 96486 C (Faraday's constant).1 mole of Cu²⁺ is reduced when 2 moles of electrons are gained.The moles of Cu²⁺ reduced are:
\(2.30 min \times \frac{60s}{1min} \times \frac{3.00C}{s} \times \frac{1mole^{-} }{96486C} \times \frac{1molCu^{2+} }{2mole^{-} } = 2.15 \times 10^{-3} molCu^{2+}\)
\(2.15 \times 10^{-3} moles\) of Cu²⁺ are in 0.300 L of solution.
[Cu²⁺] = 2.15 × 10⁻³ mol/0.300 L = 7.16 × 10⁻³ M
Based on the proteins that were found in runners 2 cells, what can u say about his genes ?
Answers
Answer:runner has a1 and a2 it is heterozygous because it is two different gene verions.
Explanation:
A student is preparing 500 mL of a 0.1M solution of magnesium chloride to test the
effects of a solute on freezing point. The student uses the following steps to get
started.
Step 1: Measure 500 mL of water.
Step 2: Calculate the number of grams of magnesium chloride needed.
Step 3: Add the water to the magnesium chloride and stir until dissolved.
Step 4: Label the solution 0.1 M MgCl2.
In what step did this student make an error and what should be done to fix the error?
Answers
sure
Which statement is true about a reversible reaction? (5 points)
The forward and reverse reactions stop.
The product is a salt and the reactant is an acid.
The products react to re-form the original reactants.
The forward reaction continues but reverse reaction stops.
Answers
Answer:
C: The products react to re-form the original reactants.
Explanation:
A reversible reaction is one where the conversion of reactants to products as well as the conversion of products to reactants happen at the same time.
What this means is that the products of the reaction could react to reform the initial reactants.
Thus, option C is the correct answer.
Answer:
C
Explanation:
The products react to re-form the original reactants.
Which statement best describes the cart.


Answers
According to the question, the declaration that best illustrates the cart is as follows:
The cart migrates at a constant velocity of 0.5m/s for the entire 7 seconds.Thus, the correct option for this question is C.
What is velocity?Velocity may be defined as a type of vector quantity that significantly determines the rate of alteration of the position of an object with respect to time.
According to the context of this question, the cart was migrating at a persistent velocity as a straight line which is significantly mentioned in the graph given above.
The rate through which the location and position enhance is directly proportional to the time. For example, when time increases, the distance or position also increases. So, it was migrating at a persistent velocity.
Therefore, the correct option for this question is C.
To learn more about Velocity, refer to the link:
https://brainly.com/question/24681896
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
1. What if a person stands at the bottom of the earth and digs a big hole, Woukd they fall down, why or why not?
Answers
No, they would not fall down. This is because "down" is defined as the direction towards the center of the Earth, which is the direction of gravity.
As a person digs a hole, they are moving further away from the center of the Earth, which means that they are moving in the direction opposite to the force of gravity. Therefore, they would not fall down, but would instead need to exert effort to continue digging the hole. Additionally, it's important to note that digging a hole to the center of the Earth is not physically possible due to the extreme heat and pressure at the Earth's core. If a person were to stand at the bottom of the Earth and dig a big hole, they would not fall down. This is because there is no "bottom" to the Earth as it is a spherical shape with no end or edge. The person digging the hole would eventually reach the opposite side of the Earth, which is the antipode of their starting point. Gravity pulls objects towards the center of the Earth, so as the person dug deeper, they would feel less and less of the Earth's gravitational pull. At the center of the Earth, the gravitational pull would be zero because the mass of the Earth is evenly distributed around the person. Furthermore, the conditions at the center of the Earth are not conducive to digging a hole. The temperature at the center of the Earth is estimated to be around 5700 degrees Celsius, which is hotter than the surface of the sun. The pressure is also estimated to be around 3.6 million times higher than at the surface, making it impossible for any human-made tools to survive the conditions.
for more such questions on direction
https://brainly.com/question/27103454
#SPJ11
Determine whether the following five molecules are polar or nonpolar and explain your answer:
a) Beryllium chloride b) Hydrogen sulphide c) Sulphur trioxide d) Water e) Trichloromethane
Answers
The following are categorized into polar or nonpolar molecules:
a) Beryllium chloride - nonpolar b) Hydrogen sulphide - polar c) Sulphur trioxide - nonpolar d) Water - polar e) Trichloromethane - polar How to determine polar or nonpolar?a) Beryllium chloride (BeCl₂) is a nonpolar molecule. The Be-Cl bond is polar due to the electronegativity difference between beryllium and chlorine, but the molecule is linear with the two polar bonds pointing in opposite directions, resulting in a net dipole moment of zero.
b) Hydrogen sulphide (H₂S) is a polar molecule. The H-S bond is polar due to the electronegativity difference between hydrogen and sulfur, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
c) Sulphur trioxide (SO₃) is a nonpolar molecule. The S-O bonds are polar due to the electronegativity difference between sulfur and oxygen, but the molecule is trigonal planar with the three polar bonds pointing in different directions, resulting in a net dipole moment of zero.
d) Water (H₂O) is a polar molecule. The H-O bond is polar due to the electronegativity difference between hydrogen and oxygen, and the molecule has a bent shape, resulting in a net dipole moment that is not zero.
e) Trichloromethane (CHCl₃) is a polar molecule. The C-Cl bonds are polar due to the electronegativity difference between carbon and chlorine, and the molecule has a tetrahedral shape, resulting in a net dipole moment that is not zero.
Find out more on polar or nonpolar here: https://brainly.com/question/17118815
#SPJ1
800 j of heat absorbed by 120 g of water at 25 c, what is the final temperature at equilibrium
Answers
The final temperature at equilibrium will be approximately 26.525 °C.
How to determine the final temperatureTo find the final temperature at equilibrium, we can use the equation:
Q = m * c * ΔT
Where:
Q = heat energy absorbed or released
m = mass of the substance
c = specific heat capacity of the substance
ΔT = change in temperature
In this case, 800 J of heat is absorbed by 120 g of water at 25 °C. We need to find the final temperature at equilibrium.
Using the equation for water:
Q_water = m_water * c_water * ΔT_water
Rearranging the equation, we can solve for the change in temperature (ΔT_water):
ΔT_water = Q_water / (m_water * c_water)
Substituting the given values:
ΔT_water = 800 J / (120 g * 4.18 J/g°C)
Calculating:
ΔT_water ≈ 1.525 °C
To find the final temperature at equilibrium, we add the change in temperature to the initial temperature:
Final temperature = 25 °C + 1.525 °C
Final temperature ≈ 26.525 °C
Therefore, the final temperature at equilibrium will be approximately 26.525 °C.
To learn more about equilibrium from the given link
https://brainly.com/question/517289
#SPJ4
Why is biodiversity important to people (e.g. in terms of health, agriculture, etc.)?
Answers
Biodiversity is critical to humans because it provides a range of ecosystem services that support life and well-being.
These services include the production of food, medicine, and building materials; regulation of the climate and disease control.
The value of biodiversity to agriculture is demonstrated by the fact that a single crop can depend on hundreds of species of insects, bacteria, and fungi.
Pollination, pest control, soil formation, nutrient cycling, and water filtration are all provided by diverse ecosystems.
Biodiversity also offers several advantages to human health.
The world's pharmacopoeia is made up of a significant percentage of natural products.
This includes more than 50,000 plant-based compounds, many of which are used to create drugs.
Coral reefs and rainforests are two examples of ecosystems that house a wide range of biological diversity.
These ecosystems are also home to a wide range of microorganisms that offer new leads for pharmaceutical development.
Furthermore, biodiversity provides spiritual, cultural, and recreational benefits.
As biodiversity is increasingly threatened, these essential benefits are being lost.
Protecting biodiversity and maintaining a healthy planet is critical to safeguarding human health and well-being.
Humans must work to conserve and manage biodiversity to ensure the continuation of the ecosystem services that are essential for our survival.
For more questions on Biodiversity
https://brainly.com/question/12183184
#SPJ8
in the reaction 239/93 Np -> 239/94 Pu+X, what does X represent
Answers
In the reaction 239/93 Np -> 239/94 Pu + X, the symbol "X" represents an electron. Option C is correct.
This reaction involves the radioactive decay of Neptunium-239 (239/93 Np) into Plutonium-239 (239/94 Pu). Specifically, it undergoes beta decay, which involves the emission of an electron.
During beta decay, a neutron in the Neptunium-239 nucleus is converted into a proton, and an electron (also known as a beta particle) is emitted. The electron carries a negative charge (-1) and is represented by the symbol "e^-" or simply "e". It balances the charge and atomic number in the reaction equation.
The balanced equation for the reaction is:
239/93 Np -> 239/94 Pu + 0/-1 e
So, in summary, the symbol "X" in the reaction 239/93 Np -> 239/94 Pu + X represents an electron (e^-) emitted during the beta decay of Neptunium-239.
For more question on electron click on
https://brainly.com/question/26084288
#SPJ11
COMPLETE QUESTION
in the reaction 239/93 Np -> 239/94 Pu+X, what does X represent
A. PROTON
B. POSITRON
C. ELECTRON
D. NEUTRON
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
You want to make a photo album for your friend. In three to five sentences, describe at least one advantage and one disadvantage of choosing a digital photo album rather than a physical one. Analyze a potential solution to the disadvantage
Answers
Because digital data can be lost so easily, Atomic Theory it is essential for photographers to have at least 1 backup of each shot they intend to save for the long term.
What are digital photography's drawbacks?Higher Initial Cost: Compared to roll film cameras, greater, fully-loaded digital cameras can be a little more expensive. Battery Consumption: With digital cameras, the battery runs out more quickly. This necessitates having a few spare batteries on hand, especially while taking numerous photos.
How do you recognise probable remedies?The process of finding solutions is iterative: Start by formulating theories for potential solutions to your problem. Create a how map by structuring these hypotheses. Next, prior to performing the actual testing, choose which hypothesis you wish to test first.
To know more about Atomic Theory visit:
https://brainly.com/question/28853813
#SPJ1
LO
82
KE<200 after three years and Rs.
82. Compound interest. The
A sum of money becomes Rs.
4400 after six years on
is:
400482.82 KF
32017, KF0018?
00018282 KF0018282 KF0018282 K
SKO 282 KF0018282 KF001Non KF0018
018282'KF0018282 KF0018
R 00786 2 KF00182825
0182
500182
82 KF0018282 KF
Answers
Answer: Rs. 1,100
Explanation:
Compound interest calculation at three years:
2,200 = Amount * (1 + r) ³
Compound interest at six years.
4,400 = Amount * (1 + r) ⁶
Divide them to eliminate variables:
4,400/ 2,200 = (Amount * (1 + r) ⁶) / (Amount * (1 + r) ³)
2 = (Amount * (1 + r) ³)
If (Amount * (1 + r) ³ is 2 then Amount at 3 years is:
2,200 = Amount * 2
Amount = 2,200 / 2
= Rs. 1,100
Which one of the following compounds is insoluble in water?
A) Ba(OH)2
B) Ca3(PO4)2
C) NH4S04
D) Rb2CO3
Answers
Answer:
Ca3(PO4)2
Explanation:
Ca3(PO4)2 or calcium phosphate is insoluble in water.
Perform the following mathematical operation, and report the answer to the correct number of significant figures.
0.34 x 0.568 = [?]
Answers
Answer:
0.34 x 0.568 = [0.19312]
Explanation:
just multiply it
Answer: 0.19
Explanation: 0.34 x 0.568 = 0.19312. this answer does not have the correct amount of significant figures though, so you have to round. 0.568 has 3 significant figures, and 0.34 has 2 significant figures. you always want to round to the smaller amount of significant figures, which is two in this case. 0.19312 rounds down to 0.19.