A 50. 0 gram substance absorbs 968 J of energy and increases in temperature from 30. 1°C to 40. 2°C. Calculate the specific heat
Answers
The specific heat of the substance is 1.92 J/g°C, determined by using the formula for heat transfer.
The amount of heat needed to raise the temperature of one gram of a substance by one degree Celsius is known as the specific heat (c). The following formula can be used to compute it:
q = m x c x ΔT
where q = heat absorbed,
m = mass of the substance,
ΔT = change in temperature, and
c = specific heat.
Substituting the given values, we get:
968 J = (50.0 g) x c x (40.2°C - 30.1°C)
Simplifying and solving for c, we get:
c = 968 J / [(50.0 g) x (40.2°C - 30.1°C)]
c = 968 J / (50.0 g x 10.1°C)
c = 1.92 J/g°C
To know more about specific heat, refer:
https://brainly.com/question/26866234
#SPJ4
Related Questions
A substance has a volume of 20 mL and a density of 2.5 g/mL. What is its mass?
Answers
Answer:
mass = 50 gExplanation:
Density of a substance can be found by using the formula
\(Density(\rho) = \frac{mass}{volume} \)
From the question
Density = 2.5 g/mL
volume = 20 mL
To find the mass substitute the values into the above formula and solve for the mass
That's
Making mass the subject we have
mass = Density × volume
mass = 2.5 × 20
We have the final answer as
mass = 50 gHope this helps you
Answer:
mass=?,volume=20,density=2.5.
density=mass/volume.
which will be 2.5=mass/20
which is mass=2.5×20=50kg
Question 11
A material whose particle composition allows heat and electricity to pass through it easily are described as having a high
OA) conductivity
O B) density
OC) hardness
OD) weight
Answers
Answer:
Coductivity
Explanation:
Because heat conducts to them!
The number of Atoms present in one mole of an element is equal to Avogadro Number. Which One Of the Following contains the greatest number of Atoms? (1)4g He (2)46g Na (3)0.40g Ca (4) 12g He
Answers
Answer:
Thus, the element containing the greatest number of atoms is 12 g He. Thus, the correct option is (4) 12 g He. Note: The number of atoms of a compound is Avogadro's number for 1 mole of compound. The number 6.022×1023 is known as Avogadro's number.
Explanation:
(4)✔️ 12 g He
please follow me.
iammallikaAnswer:
120gram I think so this is the answer if the answer is correct plz mark me as brainliest.
If 76.4 grams of NH4Cl are used, how many grams of water would theoretically be produced?
25.70 g
42.5 g
20.67 g
38.2 g
Answers
The mass (in grams) of water, H₂O that would theoretically be produced is 25.70 g
How do I determine the mass of water that would be p roduced?We'll begin by writing the balanced equation for the reaction of NH₄Cl to produce water. This given below:
NH₄Cl + NaOH -> NH₃ + NaCl + H₂O
Molar mass of NH₄Cl = 53.5 g/molMass of NH₄Cl from the balanced equation = 1 × 53.5 = 53.5 g Molar mass of H₂O = 18 g/molMass of H₂O from the balanced equation = 1 × 18 = 18 gFrom the balanced equation above,
53.5 g of NH₄Cl reacted to produce 18 g H₂O
Now, we shall determine the mass of water, H₂O produced from the reaction of 76.4 g of NH₄Cl. Details below:
From the balanced equation above,
53.5 g of NH₄Cl reacted to produce 18 g H₂O
Therefore,
76.4 g of NH₄Cl will react to produce = (76.4 × 18) / 53.5 = 25.70 g of H₂O
Thus, the mass of water, H₂O produced is 25.70 g
Learn more about mass produced:
https://brainly.com/question/9526265
#SPJ1
Does transfer RNA perform its function in the nucleus or cytoplasm? Explain.
Answers
Answer:
Explanation:
Although tRNAs participate in the essential function of protein translation in the cytoplasm, tRNA transcription and numerous processing steps occur in the nucleus.
Transfer RNA carries amino acids to the ribosome and adds them to the growing protein, copies the coded message from the DNA in the nucleus and carries the message to the ribosome in the cytoplasm.
Given the following equation: 8 Fe + Sg 8 FeS What mass of iron is needed to react with 16. 0 grams of sulfur?
Answers
223.13 grams of iron is required to react with 16.0 grams of sulfur.
Chemical equation: 8 Fe + Sg → 8 FeS
Molar mass of iron (Fe) = 55.85 g/mol
Molar mass of sulfur (Sg) = 32.07 g/mol
To determine the mass of iron required to react with 16.0 grams of sulfur, we follow these steps:
Step 1: Calculate the number of moles of sulfur (Sg):
Number of moles of Sg = Mass of Sg / Molar mass of Sg
Number of moles of Sg = 16.0 g / 32.07 g/mol
Number of moles of Sg = 0.499 moles
Step 2: Calculate the number of moles of iron (Fe) required to react with sulfur:
Number of moles of Fe = Number of moles of Sg × (8 moles of Fe / 1 mole of Sg)
Number of moles of Fe = 0.499 mol × (8 mol / 1 mol)
Number of moles of Fe = 3.99 moles
Step 3: Calculate the mass of iron needed:
Mass of iron = Number of moles of Fe × Molar mass of Fe
Mass of iron = 3.99 mol × 55.85 g/mol
Mass of iron = 223.13 g
Therefore, 223.13 grams of iron is required to react with 16.0 grams of sulfur.
Learn more about sulfur
https://brainly.com/question/1478186
#SPJ11
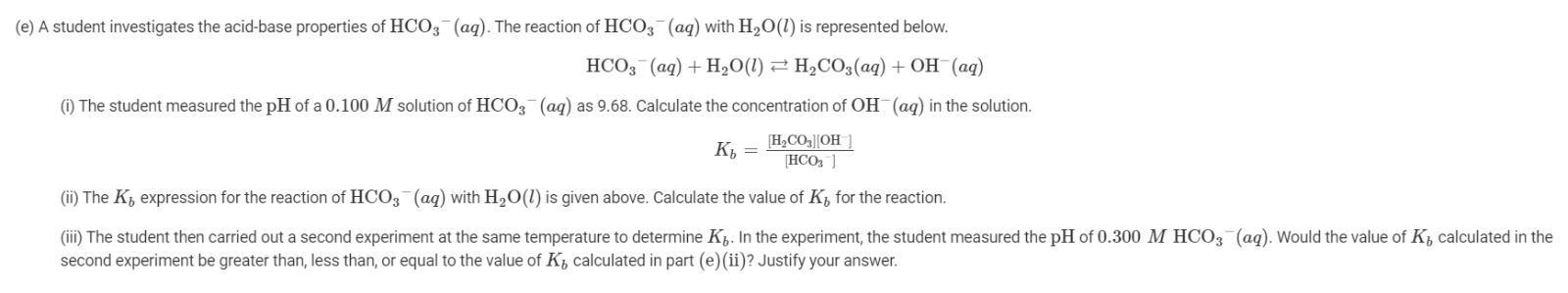
a. Two possible Lewis electron-dot diagrams for CO2 are shown above. Explain in terms of formal charges why diagram Z is the better diagram.
b. Identify the hybridization of the valence orbitals of the C atom in the CO2 molecule represented in diagram Z.



Answers
The carbon atom is SP hybridized while the oxygen atom is SP2 hybrized.
What are resonance structures?The term resonance structures are those structures that can be used to explain the bonding in a molecule. Sometimes, a single structure is insufficient in explaining the structure of a compound.
Now looking at the two sturctures, Z is better because there are mo formal charges which make the molecle less stable unlike in X. In the CO2 molecule, the carbon atom is SP hybridized while the oxygen atom is SP2 hybrized.
Learn kire about hydridization of atoms: https://brainly.com/question/5637463
If the mass of an object is 200 kg and the applied force is 2,600 N, calculate the acceleration.
Answers
Answer:
a = 13 m/s²
Explanation:
Given data:
Mass of object = 200 Kg
Applied force = 2600 N
Acceleration = ?
Solution:
Definition:
The acceleration is rate of change of velocity of an object with respect to time.
Formula:
a = Δv/Δt
a = acceleration
Δv = change in velocity
Δt = change in time
Units:
The unit of acceleration is m.s⁻².
Acceleration can also be determine through following formula,
F = m × a
a = F/m (N = kgm/s²)
a = 2600 kgm/s² / 200 Kg
a = 13 m/s²
when 81.9 g of calcium and 40.0 g of nitrogen gas undergo a reaction that has a 80.0% yield, what mass of calcium nitride forms
Answers
Answer:
309.38 g
Explanation:
To determine the mass of calcium nitride that forms when 81.9 g of calcium and 40.0 g of nitrogen gas undergo a reaction with an 80.0% yield, use the mass of the reactants and the chemical formula for calcium nitride to determine the theoretical yield of the reaction.
The first step
Determine the number of moles of calcium and nitrogen present in the reactants. Do this by dividing the mass of each element by its atomic mass ( found on the periodic table).
calcium, the atomic mass is 40.08 g/mol, so the number of moles of calcium present is
81.9 g / 40.08 g/mol = 2.05 moles.
nitrogen, the atomic mass is 14.01 g/mol, so the number of moles of nitrogen present is
40.0 g / 14.01 g/mol = 2.86 moles.
The second step
use the chemical formula for calcium nitride (Ca3N2) to determine the mole ratio between the reactants and the product. In this case, for every 3 moles of calcium, 2 moles of nitrogen are needed to form calcium nitride.
Therefore
Use this mole ratio to determine the number of moles of calcium nitride that would be produced by the reaction if the yield were 100%. To do this, divide the number of moles of each reactant by the mole ratio to determine the number of moles of calcium nitride produced.
For calcium,
we have 2.05 moles / 3 moles/mol of Ca3N2 = 0.68 moles of Ca3N2.
For nitrogen,
we have 2.86 moles / 2 moles/mol of Ca3N2 = 1.43 moles of Ca3N2.
Since the yield of the reaction is 80%, multiply the theoretical yield (which is the amount of calcium nitride that would be produced if the yield were 100%) by the yield to determine the actual amount of calcium nitride that is produced.
In this case, the theoretical yield of the reaction is 0.68 moles + 1.43 moles = 2.11 moles of Ca3N2.
The actual yield of the reaction is 2.11 moles * 80% = 1.69 moles of Ca3N2.
Finally,
convert the number of moles of calcium nitride to grams by multiplying the number of moles by the molar mass of calcium nitride (which is 183.02 g/mol).
The mass of calcium nitride produced is 1.69 moles * 183.02 g/mol = 309.38 g .
therefore, if 81.9 g of calcium and 40.0 g of nitrogen gas react forming a reaction that has an 80.0% yield, 309.38 g of calcium nitride will form.#SPJ4
The force of gravity is dependent on certain factors. According to the illustration, what factor(s) influence the gravitational attraction between two objects. A) mass and distance B) distance and pull C) composition and mass D) mass and density

Answers
According to the illustration, the factors that influence the gravitational attraction between two objects would be mass and distance. Option A.
Factors influencing gravitational attractionThe gravitational attraction between two objects is determined by two factors: their masses and the distance between them.
The greater the mass of each object, the greater the gravitational attraction between them. The farther apart the objects are, the weaker the gravitational force between them.This is due to the inverse square law, which states that the force between two objects is proportional to the inverse square of the distance between them.
Therefore, the correct answer is mass and distance.
More on gravitational attraction can be found here: https://brainly.com/question/29009418
#SPJ1
Answer:it is A
Explanation:
Compare the montreal (blue curve), london (orange curve), and beijing (purple curve) protocols. which protocol is the least aggressive at decreasing stratospheric chlorine emissions?
Answers
The Montreal Protocol, London Protocol, and Beijing Protocol are international agreements aimed at reducing stratospheric chlorine emissions and protecting the ozone layer. Among these protocols, the least aggressive in terms of decreasing stratospheric chlorine emissions is the Montreal Protocol.
The Montreal Protocol, adopted in 1987, is a global treaty that regulates the production and consumption of ozone-depleting substances (ODS). It has been successful in phasing out the production of major ODS such as chlorofluorocarbons (CFCs) and halons. By reducing the use of these substances, the Montreal Protocol has effectively decreased stratospheric chlorine emissions and helped in the recovery of the ozone layer.
The London Protocol, adopted in 1990, is an amendment to the original Montreal Protocol. It focuses on the elimination of chemicals known as ozone-depleting substances (ODS). Although it has contributed to the reduction of stratospheric chlorine emissions, the London Protocol is not as aggressive as the Montreal Protocol in terms of its targets and timelines for phase-out.
To know more about chlorine emissions visit:-
https://brainly.com/question/31560014
#SPJ11
The mouth, esophagus, stomach, and the intestines belong to what body system?
A. reproductive
B. digestive
C. respiratory
D. circulatory
E.muscular
Answers
Answer:
B. Digestive System
Explanation:
The hollow organs that make up the GI tract are the mouth, esophagus, stomach, small intestine, large intestine, and anus. The liver, pancreas, and gallbladder are the solid organs of the digestive system. The small intestine has three parts. The first part is called the duodenum.
Answer:
B- Digestive
Explanation:
If you look at the diagram of the digestive system, you'll notice that the food is taken through the mouth which then goes through a pipe which is esophagus and enters the stomach. Which hence comes under the digestive system.
hope it helps :))
g. How does modern periodic law explain the cause of periodicity
Answers
Answer:
The cause of periodicity in properties is the repetition of similar outer electronic configuration after certain regular intervals. For example, all the elements of group 1 i.e. alkali metals have a similar outer electronic configuration, ns1. Here n refers to the Principal Quantum Number of the outermost shell.
Explanation:
What will the pH of 1.50 L of pure water water be if 2.0 mL of 4.0 M HCl is added? By how much has the pH changed? What will the pH of the solution in part b be if 2.0 mL of 4.0 M HCl is added? By how much has the pH changed?
Answers
Answer:
Part A
pH ≈ 2.273
Part B
ΔpH ≈ -4.726
Part C
pH ≈ 1.973
Part D
ΔpH ≈ -0.301
Explanation:
Part A
The pH of a solution is given by the negative concentration of hydrogen ions in the solution
2.0 mL = 0.002 L
The number of moles of HCl in 2.0 mL of 4.0 M HCl is given as follows;
1 Liter of 4.0 M HCl contains 4.0 moles of HCl
2.0 mL = 0.002 L 4.0 M HCl contains 0.002 L/(1 L) × 4.0 M = 0.008 moles of HCl
The concentration of 0.008 moles in 1.50 L is given as follows;
Concentration = The number of moles/(The volume in liters)
∴ The concentration of 0.008 moles in 1.50 L, C = 0.008 moles/(1.5 L + 0.002 L)
∴ The concentration of 0.008 moles in 1.50 L, C ≈ 0.00533 moles/liter = 0.00533 M HCl
Given that HCl is a strong acid, we have that HCl dissociates completely to give equal number of H⁺ and Cl⁻ ions;
The number of moles of [H⁺] in the solution = 0.00533 moles
The pH of the solution = -log[H⁺]
∴ pH = -log[5.33 × 10⁻³] ≈ 2.273
The pH of the 1.5 L of pure water will be approximately 2.273
Part B
The pH of the pure water has changed from neutral (pH = 7) tp pH = 2.273
The change in pH is ΔpH = 2.274 - 7 = -4.726
ΔpH ≈ -4.726
Part C
When 2.0 mL of the 4.0 M HCl is added, the solution above, we have;
C = (0.008 + 0.008)/(1.5 + 0.002 + 0.002) ≈ 1.06383 × 10⁻²
The concentration of the solution becomes, C ≈ 1.06383 × 10⁻² mole/liter
The pH becomes, pH = -log(1.06383 × 10⁻²) ≈ 1.973
Part D
The amount by which the pH has changed, ΔpH ≈ 1.973 - 2.274 = -0.301.
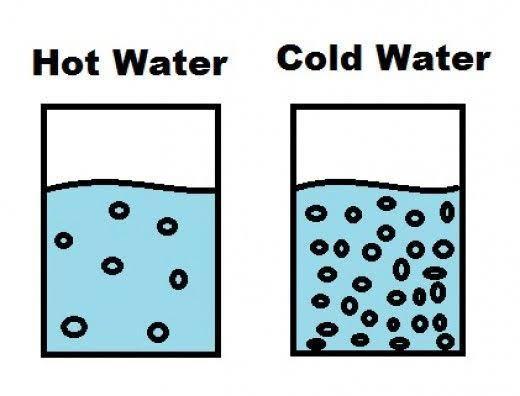
3- You saw a jar of hot water placed upside down over a jar of cold water. The hot
water stayed on top of the cold water without mixing. Why did the hot water stay on
top of the cold water?
Answers
Hot water stays on the top of cold water and doesn't mix. This is because of difference in DENSITY of water at different temperatures.
When we heat water, then the atoms gain energy from the heat and start moving away from each other. Now, when the atoms move away from each other than the (mass per unit volume) i.e. the density decreases. This makes water lighter.
When the temperature is less i.e. cold water then the atoms are more compactly packed and therefore the density of water is more at lower temperatures.
Since, hot water is lighter than cold water that is why it stays on top of the cold water.
What subatomic particles vary between isotopes of an element?
Answers
Answer:
The atoms of a chemical element can exist in different types. These are called isotopes. They have the same number of protons (and electrons), but different numbers of neutrons.
Explanation:
Answer:
The atoms of a chemical element can exist in different types. These are called isotopes. They have the same number of protons (and electrons), but different numbers of neutrons.
Explanation:
calcium reacts with water to form calcium hydroxide and hydrogen. in the balanced equation for this reaction, what is the coefficient of hydrogen?
Answers
When calcium reacts with water to form calcium hydroxide and hydrogen in the balanced equation for this reaction, then the coefficient of hydrogen will be 1.
Calcium is a chemical element having symbol Ca and its atomic number will be 20. As an alkaline earth metal, calcium is a very reactive metal that forms a dark oxide-nitride layer when it is exposed to air. Its physical as well as chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminum.
The balanced chemical equation for the reaction of calcium with water to form calcium hydroxide and hydrogen is:
Ca + 2H₂O → Ca(OH)₂ + H₂
In this equation, the coefficient of hydrogen (H₂) is 1.
To know more about calcium here
https://brainly.com/question/4023397
#SPJ4
if the ph of a salt solution was determined to be 5.89, what is the concentration of hydronium present in the solution. input your answer in decimal format, not scientific notation, and be sure to round to the appropriate number of significant figures
Answers
The concentration of hydronium ions in the solution is approximately 1.41 × 10⁻⁶ M.
What is concentration of ions in a solution?The concentration of ions in a solution depends on the specific ions present and their stoichiometry. In a salt solution, the concentration of ions can be determined based on the dissociation of the salt.
For example, if we have a salt NaCl, it dissociates in water to form Na+ and Cl- ions. The concentration of Na+ ions and Cl- ions would be equal and can be calculated based on the molar concentration of the salt solution.
If we have a 1 M NaCl solution, the concentration of Na+ ions and Cl- ions would also be 1 M.
In general, the concentration of ions in a solution can be determined by knowing the concentration of the compound that dissociates into those ions and considering the stoichiometry of the dissociation reaction.
The pH of a solution is a measure of the concentration of hydronium ions [\(\rm H_3O^+\)] . The pH scale ranges from 0 to 14, where a pH of 7 is considered neutral, pH below 7 is acidic, and pH above 7 is basic.
To determine the concentration of hydronium ions in a solution from its pH, we can use the equation:
[\(\rm H_3O^+\)] = \(10^{(-pH)\)
Given that the pH of the salt solution is 5.89, we can calculate the concentration of hydronium ions:
[\(\rm H_3O^+\)] = \(10^{-5.89}\)
[\(\rm H_3O^+\)] ≈ 1.41 × 10⁻⁶
Therefore, the concentration of hydronium ions in the solution is approximately 1.41 × 10⁻⁶ M.
To know more about concentration of ions, refer here:
https://brainly.com/question/29737756
#SPJ4
A. Observe: What do you
think is gonna happen to
these two magnets ?
Answers
Answer:
opposites attract, the same repel each other
Explanation:
The process of generating electricity from dams and nuclear power plants is very different, but some of the energy transformations are the same. Which energy transformations occur in both dams and nuclear power plants? Check all that apply.
Answers
Answer:
Kinetic energy to mechanical energy, and mechanical energy to electrical energy.
Explanation:
From the Law of conservation of Energy, which state that energy can neither be created nor destroyed but can be transformed from one form to another. Energy transformation is essential in science and technology, in the process of generating electricity from dams and nuclear power plants
some of the energy transformations are the same, the energy transformations occur in both dams and nuclear power plants is Kinetic energy to mechanical energy, and mechanical energy to electrical energy. Kinectic energy is the energy in motion which means the dams is a running water and posses a Kinectic energy then it's is been convert to mechanical energy (which is the macroscopic energy) then to electrical energy by producing light.
Answer:
Explanation:
Kinetic energy to mechanical and mechanical to electrical
which mineral would most likely be found in a necklace? graphite, halite, sulfur, or emerald?
Answers
Answer:
D is the answer because I think it is right plus I know they don't use two off them
Answer:Emerald is a gemstone that might be found in a necklace.
Explanation:Trust me i just got it right and i get all my other questions right.
How many moles of C are needed to produce 4.5 moles of CO?
How many grams of antimony (Sb) are produced when 56.7 grams of antimony oxide (Sb2O3) are used up completely?
A chemist is able to collect 18.3 grams of Sb from the reaction of 42 grams of C with excess Sb2O3. What is the chemist’s percent yield of Sb?
A chemist has 62 grams of Sb2O3 and 33 grams of C available to produce Sb. What is the limiting reagent?
Answers
Answer: 2.25 moles of C, 40.6 grams of Sb, 77%, and
How many moles of C are needed to produce 4.5 moles of CO?Answer: 2.25 moles of C
Explanation: To answer this question, we need to use a mole ratio, which is a stoichiometric relationship between the amounts in moles of any two substances in a chemical reaction. The balanced equation for the reaction is:
\ce {C} (s) + \ce {O2} (g) \rightarrow \ce {CO} (g) + \ce {CO2} (g) C(s) + OX 2(g) → CO(g) + COX 2(g)
The mole ratio between C and CO is 1:1, which means that for every mole of C that reacts, one mole of CO is produced. Therefore, to produce 4.5 moles of CO, we need 4.5 moles of C.
How many grams of antimony (Sb) are produced when 56.7 grams of antimony oxide (Sb2O3) are used up completely?Answer: 40.6 grams of Sb
Explanation: To answer this question, we need to use composition stoichiometry, which is the calculation of quantities by weight in a reaction described by a balanced equation. The balanced equation for the reaction is:
\ce {Sb2O3} (s) + \ce {C} (s) \rightarrow \ce {Sb} (s) + \ce {CO} (g) SbX 2OX 3(s) + C(s) → Sb(s) + CO(g)
To find the mass of Sb produced from a given mass of SbX 2OX 3, we need to convert the mass of SbX 2OX 3 to moles using its molar mass, then use the mole ratio between SbX 2OX 3 and Sb to find the moles of Sb produced, and then convert the moles of Sb to mass using its molar mass. The molar masses of SbX 2OX 3 and Sb are 291.5 g/mol and 121.8 g/mol, respectively. The mole ratio between SbX 2OX 3 and Sb is 1:2, which means that for every mole of SbX 2OX 3 that reacts, two moles of Sb are produced.
A chemist is able to collect 18.3 grams of Sb from the reaction of 42 grams of C with excess Sb2O3. What is the chemist’s percent yield of Sb?Answer: 77%
Explanation: To answer this question, we need to use the concept of percent yield, which is the ratio of the actual yield (the amount of product obtained from a reaction) to the theoretical yield (the maximum amount of product that can be obtained from a reaction) expressed as a percentage. The balanced equation for the reaction is:
\ce {Sb2O3} (s) + \ce {C} (s) \rightarrow \ce {Sb} (s) + \ce {CO} (g) SbX 2OX 3(s) + C(s) → Sb(s) + CO(g)
A chemist has 62 grams of Sb2O3 and 33 grams of C available to produce Sb. What is the limiting reagent?Answer: C is the limiting reagent
Explanation: To answer this question, we need to use the concept of limiting reagent, which is the reactant that is completely used up in a reaction and thus determines when the reaction stops. The balanced equation for the reaction is:
\ce {Sb2O3} (s) + \ce {C} (s) \rightarrow \ce {Sb} (s) + \ce {CO} (g) SbX 2OX 3(s) + C(s) → Sb(s) + CO(g)
To find the limiting reagent, we need to compare the amounts of SbX 2OX 3 and C in moles and use the mole ratio between them from the balanced equation. The mole ratio between SbX 2OX 3 and C is 1:1, which means that for every mole of SbX 2OX 3 that reacts, one mole of C is needed. Therefore, if we have more moles of one reactant than the other, that reactant is in excess and the other one is limiting. To find the moles of SbX 2OX 3 and C, we need to use their molar masses. The molar masses of SbX 2OX 3 and C are 291.5 g/mol and 12.0 g/mol, respectively.
Since we have more moles of C than SbX 2OX 3, C is in excess and SbX 2OX 3 is limiting. However, another way to find the limiting reagent is to use a formula based on the mole ratio:
Limiting Reagent Formula
Determine the amount of each reactant in moles.In the balanced chemical equation, divide the actual number of moles of each reactant by its stoichiometric coefficient.The limiting reactant is the one with the lowest mole ratio.Using this formula, we can find the limiting reagent.Since 0.213 < 2.75, SbX 2OX 3 is the limiting reagent.
Hope this helps, and have a great day! =)
Q
Unit
If the rules for significant figures
are observed in the addition
example shown, how should the
total for this addition be rewritten?
A. 5,610.00
B. 5,610.340
C. 5,610.34
D. 5,610.3
35.7
432.33
+ 5,142.312
5,610.342
Answers
The correct answer to the addition of the given number will be D. 5,610.3 based on the rules for addition with significant figures.
We have to find the addition of:
35.7 + 432.33 + 5,142.312 = 5,610.342
However, according to the rule for significant figures, if the addend with the fewest decimal places is 1, the final consequent will also have one decimal place. If the lowest addend is 2, the final consequent will have two decimal places, and so on.
Because significant numbers are established in the form of digits, they are also known as significant digits. The number of significant digits can be determined by counting all of the values beginning with the first non-zero digit on the left. These numbers are dependable and required to represent the quantity of a length, volume, mass, measurement, and so on.
Learn more about significant digits here:
brainly.com/question/1658998
#SPJ9
in this experiment, you qualitatively observed reactions and recorded observations. there is error associated with this. meika thinks its a systematic error. select the option that best defends meika's position. a. you will carefully time each part of the experiment
b. the experimental work will consist mostly of careful observation, recorded concisely and accurately
c. your work needs to be of higher quality than you have previously demonstrated
d. you will be doing many quantitative measurements, for example weighings, etc.
Answers
Meika's position is best defended by the experimental work will consist mostly of careful observation, recorded concisely and accurately. Thus, option b is correct.
Systematic error is an error that occurs because of a constant offset from the actual value due to a flaw in the procedure or equipment.
By carefully timing each part of the experiment, you eliminate the possibility of systematic errors due to variations in timekeeping.
Therefore, Maika's position would best be defended by the experimental work will consist mostly of careful observation, recorded concisely and accurately." Although this option is a good defense for the experiment, it directly addresses Meika's position.
This statement implies that the error associated with the experiment could be related to the way the observations were made, recorded, or interpreted, which suggests a possible systematic error.
Thus, option b is correct.
Learn more about Systematic error here:
https://brainly.com/question/14639975
#SPJ11
what is the final chloride ion concentration when 34. g zncl2 is dissolved in enough water to make 696. ml of solution? the molar mass of zncl2 is 136.3 g/mol.
Answers
Final chloride ion concentration is 1.03 mol / Cl- L when 34. g of zncl2 have been dissolved in 696. ml of water.
What is chloride ion ?The anion Cl⁻ is the chloride ion. When the element chlorine gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents, it is formed. Chloride salts like sodium chloride are frequently very soluble in water.It is an electrolyte found in all bodily fluids that is responsible for maintaining acid/base balance, transmitting nerve impulses, and regulating liquid flow into and out of cells.Less frequently, the word chloride may appear in the "common" name of a chemical compound containing one or more covalently bonded chlorine atoms.First we need to convert zncl2 to moles:
34 g zncl2 x 1 mole zncl2/ 136.3 g = 0.24 mol zncl2
Given the molecular formula, we have three chlorine atoms for every mole of zncl2
0.24 mol zncl2 x 3 mole Cl-/ 1 mole zncl2 = 0.72 mol Cl-
Now we will divide this by the volume of the solution as liters
696 ml x 1 L/ 1000 mL = 0.696 L
Concentration = 0.72 mol Cl- / 0.696 L = 1.03 mol / Cl- L
To learn more about chloride ion refer :
https://brainly.com/question/11295474
#SPJ4
What type of specialized cell in the eye is used for detecting low levels of light?
rod cell
cone cell
blood cell
stem cell
Answers
Answer:
Cone Cell
Explanation:
There are about six to seven million cones in a human eye and are most concentrated towards the macula. Cones are less sensitive to light than the rod cells in the retina (which support vision at low light levels), but allow the perception of color.
You forgot to dry the bread knife when you washed it and reddish brown spots appeared on it. is it chemistry or physical change?
Answers
Answer:
Chemical because water + metal = rust.
Explanation:
A new substance was formed.
K2CrO4 is added to a solution containing lead (II) and barium ions. If a precipitate is formed, what is it?
Answers
The precipitate is barium chromate.
What is a precipitate?The term precipitate refers to a solid that separates out in an aqueous phase reaction. In this case, we have two reactants in aqueous phase which is potassium dichromate and barium ions. We know that this reactions must produce a solid product. This solid product is what we call a precipitate.
The precipitate in this case is a pure white solid that is completely insoluble in water but could dissolve slightly in an acidic solution. We have to remember also that in this case, the barium ions could be sequestrated from the system by the addition of the dichromate solution.
Let us consider the reaction equation; \(K_{2} CrO_{4} (aq) + Ba^{2+} (aq) ----- > BaCrO_{4} (s)+ 2K^{+} (aq)\). From here it is obvious that a precipitate is formed and that is barium chromate.
Learn more about precipitate:https://brainly.com/question/13850174
#SPJ1
A fire that is burning wood will release water vapor and carbon dioxide
true or false?
Answers
Answer:
true.
Explanation:
when the concentration of NADH increases while the concentration of NAD+ decreases, will the electrochemical potential increase, decrease, or remain the same
Answers
When the concentration of NADH increases while the concentration of NAD+ decreases, the electrochemical potential however will increase
What is electrochemical potential?Electrochemical potential can be defined as a measure of the difference between the average energy of the outermost electrons of the molecule or element in its two valence states.
What is NADP?NADP is a coenzyme and simply has it full meaning as Nicotinamide Adenine Dinucleotide Phosphate
Learn more about NADP and NAD+ concentration:
https://brainly.com/question/8659461