a 60.ml sample of naoh(aq) was titrated with 0.10mhcl(aq) . based on the resulting titration curve shown above, what was the approximate concentration of naoh in the sample?
Answers
The approximate concentration of naoh in the sample is given as 0.05
How to solve for titration curve?moles of HCl = concentration of HCl x volume of HCl used
Then, you can use the mole ratio between NaOH and HCl to calculate the number of moles of NaOH:
moles of NaOH = moles of HCl
Finally, you can calculate the concentration of NaOH in the sample using the following equation:
concentration of NaOH = moles of NaOH / volume of NaOH sample
0.10 x 30 / 60 ml
= 0.05
Read more on titration here:https://brainly.com/question/186765
#SPJ1`
Related Questions
Base A dissociates 25% in water. Base B dissociates 50%. Base C dissociates
75%. Which base is the WEAKEST conductor of electricity?
Answers
Answer:Base A is the weakest conductor electricity
Explanation:
Dissociation is a factor that affects electrical conductivity. The greater the percentage of dissociation for bases the stronger the conductivity of electricity.
Given that
Base A dissociates 25% in water
Base B dissociates 50%.
Base C dissociates 75%
We can conclude that Base A is the weakest conductor oelectricity since it has the lowest percentage of dissociation.
Obtain solutions of CoCl2.6H2O in EtOH and CoCl2.6H2O in H2O into test tubes. Record your observations of the initial solutions.
Answers
Answer: I don't have the ability to conduct physical experiments or obtain solutions. However, I can provide you with some general information about the properties of CoCl2.6H2O in EtOH and H2O.
Cobalt chloride hexahydrate (CoCl2.6H2O) is a crystalline solid that is soluble in both water and ethanol. When dissolved in water, it forms a pink-colored solution due to the presence of hydrated cobalt ions. In contrast, when dissolved in ethanol, it forms a blue-colored solution due to the formation of a complex between cobalt ions and ethanol molecules.
The addition of CoCl2.6H2O to water or ethanol should result in the formation of a homogenous solution with a characteristic color. The color of the solution will depend on the solvent used and the concentration of the CoCl2.6H2O solution.
Toothpaste contains Sodium fluoride which is used to prevent tooth decay. How many grams are in 1 mole NaF?
Answers
Answer:
0.24%
Explanation:
A toothpaste contains 0.24% by mass sodium fluoride (NaF) used to prevent dental caries and 0.30% by mass triclosan, C12H7Cl3O2, a preservative and antigingivitis agent. One tube contains 119 g of toothpaste. (6.4, 6.5) Components in toothpaste include triclosan and NaF.a. How many moles of NaF are in the tube of toothpaste?b. How many fluoride ions (F?) are in the tube of toothpaste?c. How many grams of sodium ion (Na+) are in 1.50 g of toothpaste?d. How many molecules of triclosan are in the tube of toothpaste?Solution 124CQStep 1 of 4:Given:Mass of toothpaste = 119 g. Mass percent of sodium fluoride (NaF) = 0.24%Mass percent of triclosan (C12H7Cl3O2) = 0.30%a. Here, we are asked to find the number of moles of NaF in the tube of toothpaste.Mass percent of sodium fluoride (NaF) = 0.24%First, let’s find the mass of NaF in the tube: = 119 g = 0.2856 grams Molar mass of NaF = 41.98 g/mol This means, 1 mol NaF = 41.98 g. So, the mole-mass factor is: and Therefore, number of moles of NaF will be: = 0.2856 g NaF = 0.00680 mol NaFHence, the number of moles of NaF in 119g toothpaste is 0.00680 moles. ________________
According to mole concept, there are 41.988 grams in 1 mole NaF that is sodium fluoride.
What is a mole?Mole is defined as the unit of amount of substance . It is the quantity measure of amount of substance of how many elementary particles are present in a given substance.
It is defined as exactly 6.022×10²³ elementary entities. The elementary entity can be a molecule, atom ion depending on the type of substance. Amount of elementary entities in a mole is called as Avogadro's number.
It is widely used in chemistry as a suitable way for expressing amounts of reactants and products.For the practical purposes, mass of one mole of compound in grams is approximately equal to mass of one molecule of compound measured in Daltons. Molar mass has units of gram per mole . In case of molecules, where molar mass in grams present in one mole of atoms is its atomic mass.
Number of moles is given by the formula, mass/molar mass and hence mass= 1×41.988= 41.988 g.
Thus, there are 41.988 g in 1 mole NaF.
Learn more about mole,here:
https://brainly.com/question/26416088
#SPJ2
A student didn't notice that her pipet tip was broken. She used the pipet properly but it consistently delivered 4. 70mL of vinegar instead of the desired 5. 00mL. How would the percent by mass results obtained have been affected? Explain your answer
Answers
The percent by mass results obtained would be higher than the actual value. This is because the mass of the vinegar delivered was consistently less than the desired amount, resulting in a lower total mass of vinegar used in the calculation.
As a result, the ratio of the mass of acetic acid (the solute) to the total mass of the solution (solute + solvent) would be higher, leading to an overestimated percent by mass value.
In order to calculate the percent by mass of acetic acid in vinegar, one needs to know the mass of acetic acid and the total mass of the solution. The volume of vinegar is not directly related to its mass, as the density of vinegar may vary. Therefore, the student's incorrect pipet, which consistently delivered less vinegar than intended, would lead to a lower total mass of vinegar used in the calculation. As a result, the calculated percent by mass of acetic acid would be higher than the actual value. This is because the ratio of the mass of acetic acid to the total mass of the solution would be higher, as the denominator (total mass) is underestimated due to the consistently lower volume of vinegar delivered.
Learn more about acetic acid here:
https://brainly.com/question/15202177
#SPJ11
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
HELP! Calculate the pH of the following solutions:
a) [OH-] = 3.1x10^-8 M
pH = 7.51
pH = 3.18
pH = 8.00
pH = 6.49
pH = 3.10
b) [H3O+] = 5.2x10^-7 M
pH = 7.72
pH = 7.00
pH = 6.28
pH = 5.27
pH = 5.20
c) [OH-] = 5.9x10^-5 M
pH = 5.00
pH = 5.90
pH = 4.23
pH = 5.59
pH = 9.77
d) [OH-] = 5.9x10^-3 M
Answers
b) is 6.28
c) is 9.77
d) ph is 11.77
Answer:
Adding base causes the pH of a solution to increase
pH is a measure of the hydrogen ion concentration of a system. Solutions with a low pH possess a high concentration of hydrogen ions, while solutions with a high pH possess a low concentration of hydrogen ions. To increase the pH of a solution, base can be added to it. Base will release hydroxide ions (OH-) in the solution which will neutralize or reduce the H+ ions concentration in the solution. This way the pH is increased.
Explanation:
What is the empirical formula for a compound if a sample contains 1. 0 g of S and 1. 5 g of O? SO SO3 S2O2 S2O3.
Answers
The empirical formula of the given compound is \(\bold{SO_3}\).
The correct option is B.
What is an empirical formula?
The empirical formula of a chemical compound is the simplest whole-number ratio of atoms contained in the substance.
Given,
1.0 g of S
1.5 g of O
To calculate the empirical formula, we will divide the masses of the elements by their atomic weight.
For sulfur
\(\bold{\dfrac{1.0}{32} =0.03125\; mol}\)
For oxygen
\(\bold{\dfrac{1.5}{16} =0.09375\; mol}\)
Now, divide the greater value of mole came by the smaller value
\(\bold{\dfrac{0.09375\; mol}{0.03125\;mol} = 3}\)
Thus, the empirical formula for the given compound is 1 for S and 3 for O
\(\bold{SO_3}\)
Learn more about empirical formula, here:
https://brainly.com/question/11588623
2. The concentration of HCI in a solution is 1 x 10^-2 mol/L. What would the pH
of the solution be?
(Hint: use pH = -log[H3O+])
A. 2
B. 3
C. 4
D. 5
Answers
A. 2
B. 3
C. 4
D. 5A. 2
B. 3
C. 4
D. 5A. 2
B. 3
C. 4
D. 5
,SJhfgaiuehrg;oiaerhgoaw
?
Answers
Answer:
nah man
Explanation:
what is the formula of the ionic compound that forms from gallium and chlorine?
Answers
Answer:
GaCl3
Explanation:
Gallium trichloride | GaCl3 |
Answer:
GaCl3
Explanation:
Which two methods do scientists use to gather information?
A. Following religious beliefs
B. Observing the natural world
C. Expressing strong opinions
D. Carrying out investigations
Answers
the two methods scientist use to gather information are
. observing the natural world
. carrying out investigation
Writing a standard formation reaction Write a balanced chemical equation for the standard formation reaction of solid chromium(III) nitrate (Cr(NO),- 0-0 0. 5 8 ?
Answers
We need to write the balanced chemical equation for the reaction that forms one mole of the compound from its constituent elements. This reaction is: Cr(s) + 3/2 N2(g) + 9/2 O2(g) → Cr(NO3)3(s)
A standard formation reaction is a chemical reaction that forms one mole of a compound from its constituent elements under standard conditions of temperature and pressure. In this case, we need to write a balanced chemical equation for the standard formation reaction of solid chromium(III) nitrate.
Firstly, we need to identify the elements present in the compound. Chromium(III) nitrate contains the elements chromium (Cr), nitrogen (N), and oxygen (O).
Next, we need to write the balanced chemical equation for the reaction that forms one mole of the compound from its constituent elements. This reaction is:
Cr(s) + 3/2 N2(g) + 9/2 O2(g) → Cr(NO3)3(s)
This equation shows that one mole of solid chromium(III) nitrate is formed from one mole of solid chromium, 1.5 moles of nitrogen gas, and 4.5 moles of oxygen gas under standard conditions.
In summary, the standard formation reaction for solid chromium(III) nitrate is:
Cr(s) + 3/2 N2(g) + 9/2 O2(g) → Cr(NO3)3(s)
To know more about Reaction visit:
https://brainly.com/question/30464598
#SPJ11
the "air" that puffs up a potato chip bag is actually what element?
Answers
The "air" that puffs up a potato chip bag is primarily composed of nitrogen gas (N₂).
Nitrogen gas is used for packaging due to its non-reactive nature. When the bags are filled with nitrogen gas, it displaces the oxygen inside, creating a controlled atmosphere. This is done to prevent the chips from getting stale. Oxygen can lead to oxidative reactions, causing the chips to go rancid or lose their crispiness. Nitrogen, being an inert gas, doesn't react with the chips or affect their taste or texture.
It acts as a protective barrier, shielding the chips from moisture and oxygen, thereby maintaining their freshness and extending their shelf life. The use of nitrogen gas ensures that the chips remain crispy and flavorful when you open the bag.
To learn more about nitrogen gas here
https://brainly.com/question/44730
#SPJ4
How can you use probability to predict the outcome of a codominant cross?
Answers
Featured snippet from the web
Calculate: Punnett squares can be used to predict probable outcomes of genetic crosses. To calculate probability, divide the number of one kind of possible outcome by the total number of all possible outcomes. For example, if you toss a coin, the chance it will land on heads is equal to 1 ÷ 2.
A mysterious element is found in its neutral state. It has an atomic number of 88. Which element is it?
radon
uranium
radium
francium

Answers
The mysterious element with atomic number 88 is Radium.
Radium is a naturally occurring radioactive element. It is chemically represented with the symbol Ra. The atomic number of this number is 88 and this is a radionuclide.
This element is formed by the decay of Uranium and Thorium. This element also exists in isotopes and its isotopes are Ra-226 and Ra-228. The Radium-226 isotope is formed as a result of the decay series of Uranium whereas Radium-228 and Radium-224 are formed as a result of the Thorium decay series.
Like elemental radium, all isotopes of Radium are radioactive. It decays and produces Radon gas, which is a highly stable Noble gas.
Thus, the mysterious element with atomic number 88 is Radium.
To know more about Rare Earth Elements, click below:
https://brainly.com/question/1415109
#SPJ1
helium-4 and helium-5 are two isotopes of helium. assuming we are talking about neutrally charged isotopes, which of the following statements would accurately compare the two isotopes. a they have a different number of electrons b they have a different number of neutrons c they have a different number of protons. d they are exactly the same because they are both helium
Answers
The statement that would compare the two isotopes of helium is b: they have a different number of neutrons
Isotopes is the word used in chemistry for the atoms of the same element that have different numbers of neutrons but consist of the same number of electrons and protons. The difference in the number of neutrons between the isotopes of an element illustrates that the various isotopes have different masses from each other.
Helium-4 and helium-5 differ in the number of neutrons as helium-4 has 2 neutrons and is the most stable isotope of helium. On the other hand, helium-5 is an unstable isotope consisting of three neutrons.
As isotopes do not differ in the number of protons and electrons; both these isotopes of helium have two protons.
To learn more about isotopes; click here:
https://brainly.com/question/364529
#SPJ4
a gaseous hydrogen and carbon containing compound is decomposed and found to contain 74.98% c and 25.02% h by mass. the mass of 245 ml of the gas, measured at stp, was 0.879 g. what is the molecular formula of the compound?
Answers
The butene chemical formula is (CH2)4 = C4H8.
How do you calculate a compound's molecular formula?Examples of carbohydrates include glucose and sucrose. Sucrose is almost exactly twice as big as glucose, despite the fact that their empirical formulas are fairly close.Some people can tell the difference between them just on taste, but it is not advisable to stroll around tasting chemicals.The best way to tell glucose from sucrose is to find their molar masses because this makes it easy to distinguish between the two different substances.A molecular compound's formula specifies the kind and number of atoms of each element that are contained within it.The molecular formula and the empirical formula are frequently the same.The chemical formula will always be some integer multiple (n) of the empirical formula.Since the empirical formula for a molecule equals n, the molecular formula is equal to n.Divide the empirical formula mass, EFM, by the compound's molar mass, MM, to obtain the integer multiple, n.n=MM(molarmass)/EFM(empiricalformulamolarmass)The empirical and molecular formulas for methane, acetic acid, and glucose, as well as different n values, are compared in Table 6.9.1. Since methane only contains one carbon atom, its empirical formula, CH4, is also its chemical formula. The empirical formula and the molecular formula are sometimes merely multiples of one another, though. Acetic acid is the main organic acid found in vinegar. It has the chemical compound C2H4O2.Glucose, a simple sugar, serves as one of the primary sources of energy for cells. It has the chemical formula C6H12O6. They both have the same empirical formula, CH2O, despite the fact that they are quite different molecules.Example
100 g of gas are used.
C = 85.63 g / 12 g/mol = 7.1358 mol when the proper atomic mass is divided by it.
H = 14.37g/1g/mol = 14.37mol
C = 1 and H = 2 divided by smaller division
Data formula for empirical research: CH2
The volume of one mole of gas is 22.4 L at STP, or 22 400 mL.
22400 mL divided by 258 mL and 0.646 g, or 56.868 g/mol, is the gaseous mass per mole.
CH2 mass in formula equals 14g/formula units in 1 mol, which is equal to 56.868 g/mol/14g/formula unit, or 4formula units per mol.
(CH2)4 = C4H8 is the butene chemical formula.
To Learn more About molecular compound refer to:
brainly.com/question/26388921
#SPJ4
If a group of 100 exposed individuals are followed for three years to measure the frequency of chronic disease, and no individuals die or are lost to follow-up, then the cumulative incidence of disease for the three-year period will equal the prevalence of disease measured on the last day of the study period. True or False
Answers
The given statement is False. The cumulative incidence of disease for the three-year period will not necessarily equal the prevalence of disease measured on the last day of the study period.
Cumulative incidence measures the number of new cases of a disease that occur within a specific time period in a population initially free of the disease.
On the other hand, prevalence measures the proportion of individuals in a population who have the disease at a particular point in time, regardless of when they developed it.
Prevalence is influenced by both the incidence and duration of the disease, while cumulative incidence only considers the occurrence of new cases. Therefore, the two measures can be different, and the statement is false.
To know more about cumulative incidence, refer here:
https://brainly.com/question/31493651#
#SPJ11
8. What is the molecular formula of a compound with the empirical formula CCIN and a molar mass of
184.5 g/mol?
Answers
C3Cl3N3 is the correct answer.
What is atomic mass?
Atomic mass is the mass of a single atom of an element, usually expressed in atomic mass units (amu). One atomic mass unit is defined as exactly 1/12 the mass of a carbon-12 atom. Atomic mass is a weighted average of the masses of all naturally occurring isotopes of an element, taking into account their relative abundances. The atomic mass of an element is important in various fields, including chemistry, physics, and biology, as it affects the element's chemical and physical properties, such as its reactivity, density, and melting point.
Molecular formula = (Empirical formula) × n
184.5 g/mol = 14 × (12.011+1.008) = Molecular formula
Therefore, C3Cl3N3 is the molecular formula
To learn more about the atomic mass , click the given link ;
https://brainly.com/question/86444
#SPJ1
how is it d?explain please?! i do not understand.please dont guess
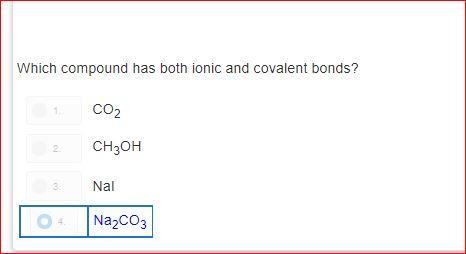
Answers
Answer:
As you can see sodium = ionic and covalent bonds aka NA 2
and CO 3 also has those two bonds the other answers dont have both numbers (bonds) Na2 and CO3 is the only answer choice that has these bonds in simpler terms ( only answer with 2 numbers)
Explanation:
List as many things as you can see in the picture below
Abiotic (Non-Living)___________Biotic (Living)
__________________ ................ ___________________ __________________ ................ ___________________
___________________ ___________________
Answers
rocks animals
water plants
air fungi
sunlight bacteria
minerals
soil
In the reaction below, which substances are products?
Choose all that apply
5 Al(s) + 24 HCl(aq) + 3 KMnO4(aq) - 5 A1C13(aq) + 3 MnCl2(aq) + 3 KCl(aq) + 8 H20(1)
-
HCl(aq)
KMnO4(aq)
H20(1)
KCl(aq)
AlCl3(aq)
Al(s)
MnCl2(aq)
Answers
Answer:
5 A1C13(aq) + 3 MnCl2(aq) + 3 KCl(aq) + 8 H20(1)
Explanation:
the products are on the right side of the arrow
What type of heat transfer do
dryers use?
Forced convection
Forced radiation
Answers
Answer:I believe it forced convections
Explanation:convection is the circular motion that happens when warmer air or liquid makes something less dense.
Answer:
how y'all doinnn
Explanation:
byyeee
Help me respond this question please

Answers
Answer:
It has two valence electrons
I NEED HELP, PLZZ
When a basketball player makes a bounce pass to another player, the bounce height is lower for each subsequent bounce and the energy of the ball decreases. What is the main reason that the bounce height decreases?
A. The energy from the pass has been destroyed.
B. The energy from the pass has been conserved.
C. The energy from the pass has been transferred to the floor.
D. The energy from the pass has been transformed into horizontal motion.
Answers
Answer:
d
Explanation:
breaking larger molecules into smaller molecules and carbon
Answers
Answer: catabolism
Explanation:
Catabolism refers to the set of metabolic pathways which is necessary for the breaking down of molecules into smaller units. This is then oxidized for the release of energy or can be used to perform other anabolic reactions.
Catabolism is regarded as the opposite direction of anabolism which is simply the building-up of molecules. It should be noted that anabolism and catabolism work together in every living organisms and perform functions such as the production of energy and the repair of cells.
Does anyone have the answers to the end of semester test B for chemistry on Edmentum?
Answers
When a solid compound is formed from chemicals that are in solution, it is called a
Answers
Answer:
Precipitate
Explanation:
Precipitate usually forms when two or more chemicals are added and a solid deposits at bottom of test tube or floats as chunky solid.
For example, when you put AgNO3(aqueous) + KCl(aqueous) —–> AgCl(precipitate) + KNO3(aqueous). You'll see a white percipitate form which AgCl.
Here's a pic of diff colors of ppt you can make

Students in Ms. Brown demonstrated several chemical reactions but the data did not represent the law of conservation of matter in
any of the four experiments. Kai and Maria are about to add 3 grams of magnesium (Mg) to a test tube of 30 grams of hydrochloric
acid (HCI). They covered their container with a balloon as a seal. If the girls expect a chernical change as well as evidence of the
Law of conservation of matter, choose ALL of the predictions that are appropriate?
A)
There will be a rapid evolution of gas.
B)
The mass of the products will equal 339.
The solid magnesium metal will disappear.
D)
The balloon covering the container will expand.
E)
There will be a dramatic change in temperature,
Answers
Answer:
Answer: everything except E
Explanation: There was no dramatic change in temperature and i just took the assignment.
According to the law of conservation of matter, matter cannot be created not be destroyed. In this experiment, no matter loss will occur but there will be rapid evolution hydrogen gas and the balloon will expand.
What is conservation of matter?According to conservation of matter, matter can neither be created nor be destroyed but its state and properties can be changed. The total mass of the reactants will be equal to the total mass of the products.
Magnesium is a second group element and is a metal. Metal reacts with hydrogen halides to form metal halides and hydrogen gas as the reaction written below:
\(Mg +2 HCl \rightarrow MgCl_{2} +H_{2}\)
The evolved hydrogen gas in this reaction tries to diffuses out the sealing and thus the balloon cover expands. And thus the solid magnesium metal disappears to form magnesium chloride.
The evolution of gases is exothermic and therefore, the temperature outside increases suddenly. There we can see bubbles of hydrogen in the reaction system.
Therefore, the changes that occur in the experiments are, rapid evolution of gases, solid metal disappears, sealing cover expands and there occur a rapid change in temperature. Thus options A,C,D,E are correct.
To learn more about of conservation of matter, refer the link below:
https://brainly.com/question/11573747
#SPJ5
h2so4(aq) + mg(s) → mgso4(aq) + h2(g) Which substance is the acid in the reaction?
Answers
Answer:
\( H_2SO_4\)
Explanation:
\( H_2SO_4\) (sulphuric acid) is the acid in the given reaction.
The acid present in the chemical equation is sulfuric acid with chemical formula H₂SO₄.
What is an acid?Acids are defined as substances which on dissociation yield H+ ions , and these substances are sour in taste.Compounds such as HCl, H₂SO₄ and HNO₃ are acids as they yield H+ ions on dissociation.
According to the number of H+ ions which are generated on dissociation acids are classified as mono-protic , di-protic ,tri-protic and polyprotic acids depending on the number of protons which are liberated on dissociation.
Acids are widely used in industries for production of fertilizers, detergents batteries and dyes.They are used in chemical industries for production of chemical compounds like salts which are produced by neutralization reactions.
Learn more about acids,here:
https://brainly.com/question/29796621
#SPJ5