Answers
Answer:
1.6 g/ml
Explanation:
To solve for the density of the liquid you must first subtract the mass of the beaker from the total mass. This comes out to 94 g - 30 g which equals 64 g. To get the density you must then divide the answer for the mass of the substance by the volume. Doing this you get 64 divided by 40, in shich you get 1.6. So the answer is 1.6 g/ml.
Related Questions
Heredity Lab Report Instructions:
In the Heredity lab, you investigated how hamsters inherit traits from their parents. Record your observations in the lab report below. You will submit your completed report.
Name and Title: Include your name, instructor's name, date, and name of lab.
Objective(s): In your own words, what was the purpose of this lab?
Hypothesis: In this section, please include the if/then statements you developed during your lab activity.
These statements reflect your predicted outcomes for the experiment.
Test One: If I breed a short fur, FF female with a short fur, Ff male, then I will expect to see (all short fur; some short and some long fur; all long fur) offspring.
Test Two: If I breed a short fur, Ff female with a short fur, Ff male, then I will expect to see (all short fur; some short and some long fur; all long fur) offspring.
Test Three: If I breed a long fur, ff female with a long fur, ff male, then I will expect to see (all short fur; some short and some long fur; all long fur) offspring.
Procedure: The procedures are listed in your virtual lab. You do not need to repeat them here.
Please be sure to identify the test variable (independent variable) and the outcome variable (dependent variable) for this investigation. Remember, the test variable is what is changing in this investigation.
The outcome variable is what you are measuring in this investigation.
Test variable (independent variable): Outcome variable (dependent variable): Data: Record the data from each trial in the data chart below. Be sure to fill in the chart completely. Test One Parent 1: FF Parent 2: Ff Phenotype ratio: ________ : ________ short fur : long fur Test Two Parent 1: Ff Parent 2: Ff Phenotype ratio: ________ : ________ short fur : long fur Test Three Parent 1: ff Parent 2: ff Phenotype ratio: ________ : ________ short fur : long fur Conclusion: Your conclusion will include a summary of the lab results and an interpretation of
Answers
For Test One, phenotype ratio is Short fur : Long fur = 2 : 0; For Test Two, the phenotype ratio is Short fur : Long fur = 3 : 1; For Test Three, the phenotype ratios will be Short fur : Long fur = 0 : 2
What are the phenotype ratios from the test crosses?For Test One:
Parent 1: FF (homozygous dominant for short fur)
Parent 2: Ff (heterozygous for short fur)
The Punnett square for this cross will give the following genotype ratios:
FF : Ff = 1 : 1
And the corresponding phenotype ratios will be:
Short fur : Long fur = 2 : 0 or 100% short fur
For Test Two:
Parent 1: Ff (heterozygous for short fur)
Parent 2: Ff (heterozygous for short fur)
The Punnett square for this cross will give the following genotype ratios:
FF : Ff : ff = 1 : 2 : 1
And the corresponding phenotype ratios will be:
Short fur : Long fur = 3 : 1 or 75% short fur and 25% long fur
For Test Three:
Parent 1: ff (homozygous recessive for long fur)
Parent 2: ff (homozygous recessive for long fur)
The Punnett square for this cross will give the following genotype ratios:
ff : ff = 1 : 0
And the corresponding phenotype ratios will be:
Short fur : Long fur = 0 : 2 or 100% long fur
For this investigation, the test variable is the breed of hamster and the outcome variable is the phenotype of the hamster.
Learn more about heredity at: https://brainly.com/question/930755
#SPJ1
When Louisa had her asthma attack, she was given oxygen through a face mask. The gauge on a 12-L tank of compressed oxygen reads 3800 mmHg. How many liters would this same gas occupy at a final pressure of 570 mmHg when temperature and amount of gas do not change?
Answers
Answer:
\(80\text{ L}\)Explanation:
Here, we want to get the volume of the gas at a reduced pressure
From Boyle's law, we have it the volume and pressure of a given mass of gas are inversely proportional
Mathematically:
\(\begin{gathered} P_1V_1\text{ = P}_2V_2 \\ \\ V_2\text{ = }\frac{P_1V_1}{P_2} \end{gathered}\)Where:
P1 is the initial pressure wich is 3800 mmHg
V1 is the initial volume which is 12 L
P2 is the final pressure which is 570 mmHg
V2 is the final volume which is ?
Substituting the values, we have it that:
\(\begin{gathered} P_2\text{ = }\frac{3800\times12}{570} \\ \\ P_2\text{ = 80 L} \end{gathered}\)It’s due in 3 minutes please help

Answers
Answer:
The Answer is gonna be Indroduction of non-native plant species
Strontium hydroxide reacts with hydrobromic acid to produce Strontium bromide and
water.
Write and balance the chemical reaction above, use it for problems 1-4 below:
1. If 5.50 moles of strontium hydroxide were consumed, how much moles of water are
produced?
2. Find the mass of hydrobromic acid used to produce 7.50 moles water.
3. If 10.8 g of strontium hydroxide were used, how much moles of strontium bromide are
produced?
4. If 13.3 g of hydrobromic acid were consumed, find the mass of the water produced.
Answers
Sr(OH)2 + 2 HCl --> SrCl2 + 2 H2O
To find the moles of water produced when 5.50 moles of strontium hydroxide are consumed, we need to apply the law of conservation of mass. The mass of water produced is equal to the mass of strontium hydroxide consumed. Since strontium hydroxide has a molar mass of 142 g/mol and water has a molar mass of 18 g/mol, 1 mol of strontium hydroxide can produce 9 mol of water. Therefore, 5.50 moles of strontium hydroxide can produce 49.5 mol of water.
Similarly, 7.50 moles of water can be produced by reacting 18 moles of hydrobromic acid with strontium hydroxide. Hydrobromic acid has a molar mass of 79.9 g/mol, so 18 moles of hydrobromic acid would have a mass of 79.9 * 18 = 1435.2 g.
To find the moles of strontium bromide produced when 10.8 g of strontium hydroxide is used, we need to apply the law of conservation of mass again. The mass of the strontium bromide produced is equal to the mass of strontium hydroxide consumed. Since strontium bromide has a molar mass of 410 g/mol and strontium hydroxide has a molar mass of 142 g/mol, 1 mol of strontium bromide can consume 3.23 moles of strontium hydroxide. Therefore, 10.8 g of strontium hydroxide can produce 10.8 / 3.23 = 3.34 moles of strontium bromide.
Finally, to find the mass of water produced when 13.3 g of hydrobromic acid is consumed, we need to apply the law of conservation of mass yet again. The mass of the water produced is equal to the mass of hydrobromic acid consumed. Since hydrobromic acid has a molar mass of 79.9 g/mol, 13.3 g of hydrobromic acid would produce 13.3 / 79.9 = 0.166 moles of water.
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
I WILL GIVE 35 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
The solution has a molarity of 0.0924 M.
What is molarity, for instance?The number of moles of solute per litre of solution is known as molarity.. For instance, water is both the solution and the solute when table salt is dissolved in it. Each mole of sodium chloride weighs 58.44 grammes. 58.44 grammes of sodium chloride are dissolved in one litre of water to produce one molar solution, or 1M.
Moles of solute per litre of solution is known as molarity (M).
Given: moles of NH3 = 0.355, volume of solution = 3.84 L
Molarity = 0.355 moles / 3.84 L = 0.0924 M
Therefore, the molarity of the solution is 0.0924 M.
To know more about molarity visit:-
https://brainly.com/question/8732513
#SPJ1
What is the total number of electrons in a S2- jon?
1.
10
2.
14
16
4.
18
Answers
the answer is D) 18 electrons
Explain in complete sentences how heat is transferred in fluids?
Answers
The process of heat transfer in fluids is known as convection and it involves the actual movement of the molecules of the fluids from hotter to cooler regions as a result of decrease in the density of the heated molecules.
What is the name given to the process of heat transfer in fluids?
The name given to the process of heat transfer in fluids is convection.
Convective heat transfer, frequently referred to as convection, is the movement of fluids that transfers heat from one location to another.
In convection, heat energy is carried by the moving fluid. The fluid moves from one area with a high temperature to another with a low temperature. In liquids and gases, it is typically the predominant type of heat transmission.
This particular technique of heat transport combines the conduction (heat diffusion) and advection processes (heat transfer by bulk fluid flow).
Learn more about convection at: https://brainly.com/question/9382711
#SPJ1
9. Which of the following gas laws is calculated with the pressure and
volume variables at a constant temperature?
Formula
4 points
P₁V₁ = P₂V₂
P₁ = first pressure
P2 = second pressure
V₁ = first volume
Answers
The gas law that is calculated with the pressure and volume variables at a constant temperature is Boyle's Law. Boyle's Law states that the pressure (P) of a gas is inversely proportional to its volume (V) when temperature (T) is held constant.
Mathematically, it is expressed as P₁V₁ = P₂V₂, where P₁ and V₁ represent the initial pressure and volume, and P₂ and V₂ represent the final pressure and volume.According to Boyle's Law, if the volume of a gas is reduced while keeping the temperature constant, the pressure will increase proportionally.
Similarly, if the volume is increased, the pressure will decrease. This relationship holds as long as the temperature remains constant throughout the process. Boyle's Law is one of the fundamental gas laws and provides insights into the behavior of gases under changing pressure and volume conditions at a constant temperature.
For more such questions on gas law
https://brainly.com/question/30233942
#SPJ11
The combustion of liquid ethanol (C2H, OH)produces carbon dioxide and water. After 5.8 mLof ethanol (density = 0.789 g/mL) was allowed toburn in the presence of 12.5 g of oxygen gas, 3.10mL of water (density = 1.00 g/mL) was collected.Part A: Determine the limiting reactantPart B. Determine the theoretical yield of H2O
Answers
A) Ethanol is the Limiting reactant
B) 5.36 grams
Explanations:The chemical reaction for the chemical combustion of ethanol is expressed as:
\(C_2H_5OH(l)+3O_2(g)\rightarrow2CO_2(g)+3H_2O(l)\)Determine the mass of ethanol
Mass of ethanol = density * volume
Mass of ethanol =0.789 * 5.8
Mass of ethanol = 4.58 grams
Determine the mass of Oxygen
Mass of Oxygen = 12.5 grams
Determine the mole of ethanol and oxygen
\(\begin{gathered} mole\text{ of ethanol}=\frac{mass}{molar\text{ mass}}=\frac{4.58}{46.07} \\ mole\text{ of ethanol}=0.0993moles \\ \end{gathered}\)For the mole of oxygen
\(\begin{gathered} mole\text{ of oxygen}=\frac{12.5}{32} \\ mole\text{ of oxygen}=0.3906mole \\ for\text{ 1 atom: }\frac{0.3906}{3}=0.1302mole \end{gathered}\)Since the mole of ethanol is lower than that of oxygen, hence the limiting reactant is ethanol
Part B: According to stoichiometry, 1mole of ethanol produces 3 moles of water, the mole of water required will be expressed as:
\(\begin{gathered} mole\text{ of water}=3\times0.0993 \\ mole\text{ of water}=0.2979moles \\ Mass\text{ of water produced}=mole\times molar\text{ mass} \\ Mass\text{ of water produced}=0.2979\times18=5.36grams \end{gathered}\)Therefore the theoretical yield of H2O is 5.36grams
The figure below shows the rotation curves of several spiral galaxies, extending to the very edge of their visible disks. What do these curves tell us about the distribution of dark matter in these galaxies?
Group of answer choices
Most of the dark matter is in the bulge.
Most of the dark matter is in the galaxies’ outskirts.
Most of the dark matter is near stars.
Most of the dark matter is in clumps.
Answers
The thing that the curves tell us about the distribution of dark matter in these galaxies is option B: Most of the dark matter is in the galaxies’ outskirts.
Why do galaxy rotation curves suggest the existence of dark matter?Beyond the visible borders of galaxies, the velocity of stars remains relatively constant, suggesting that there must be more matter present than what we can see as stars and gas.
When the impact of DE became noticeable, the rotation curves are shown to dip at a specific distance. r. These flat, v = constant, rotation curve data suggest that the amount of dark matter is increasing linearly with r up to great distances from the spiral galaxies' centers.
From the graph you can see they all pointing or going one direction, hence we can deduce that it is option B.
Learn more about dark matter from
https://brainly.com/question/28256017
#SPJ1
Explain what is cellular growth and repair? Why is it important?
Answers
Answer:
Cell growth usually refers to cell proliferation, the increase in cell numbers that occurs through repeated cell division. Cell growth can also refer to the enlargement of cell volume, which can take place in the absence of cell division. As living things grow, some cells die or become damaged and need replacements. Some single-celled organisms use a type of mitosis as their only form of reproduction. In multicellular organisms, cell division allows individuals to grow and change by expanding the number of total cells.
Hope this helps!!!
which of the following are the correct formulas for potassium oxide and calcium oxide respectively
Answers
Explanation:
potassium oxide is K2O
calcium oxide is CaO
calcium oxide is used to make glass
potassium oxide is used in fertilizer
Please help me with this thank you so much !!

Answers
Answer:
C and D
Explanation:
The answer is C and D
What is the mass of a baby (in grams) if the babies weight is 6 lbs. 6 oz?
Answers
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
When you increase the amount of carbon dioxide in the atmosphere, you also increase the amount of _____ in the ocean.
Answer: Carbonic acid
Answers
When you increase the amount of carbon dioxide in the atmosphere, you also increase the amount of carbonic acid in the ocean.
What happens when you increase the amount of carbon dioxide?Carbon dioxide is absorbed from the atmosphere into the ocean through a process called "oceanic uptake," which is facilitated by the exchange of gases at the air-sea interface.
As more carbon dioxide is emitted into the atmosphere, the concentration of carbon dioxide in the ocean increases, leading to a phenomenon called "ocean acidification".
Ocean acidification can have a number of negative impacts on marine organisms, including reduced growth rates and weakened shells or skeletons.
Thus, we can conclude this increases the amount of carbonic acid in ocean.
Learn more about increase in carbon dioxide here: https://brainly.com/question/28157529
#SPJ1
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
HEY COULD SOMEONE HELP ME WITH THIS PLS PLS ITS DUE TODAY PLS

Answers
b) gloves and other bodily protection
c) a symbol showing that it is flammable
d) they most likely were unaware. I do not think they would intentionally hurt people.
Help on both questions ? I’ll give Brainliest ( it don’t look like I spelled it right but I think I did )

Answers
Answer:
friction slows motion
Explanation:
sorry I don't know the other one
(The Question is in the photo Sorry ) If there are 5 liters of oxygen gas (O2) at 300K and 400 torr, what will it weigh?Answer in units of g
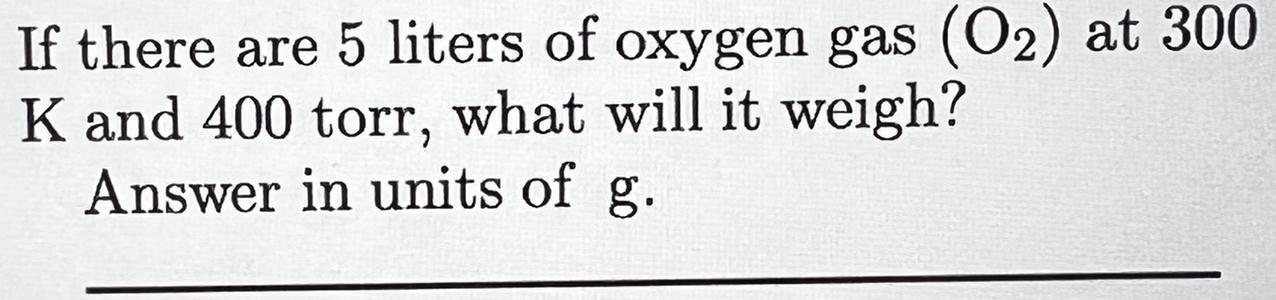
Answers
To find the mass of oxygen we have to use the ideal gas law to find the number of moles of gas at the given conditions.
First, convert the pressure in torr to atm:
\(400torr\cdot\frac{1atm}{760torr}=0.53atm\)Use the ideal gas law and solve it for n:
\(\begin{gathered} P\cdot V=n\cdot R\cdot T \\ n=\frac{P\cdot V}{R\cdot T} \end{gathered}\)Replace for the known values (R, the ideal gas constant, has a value of 0.082atmL/molK):
\(\begin{gathered} n=\frac{0.53atm\cdot5L}{0.082\cdot\frac{atmL}{molK}\cdot300K} \\ n=0.108mol \end{gathered}\)Finally use the molecular mass of oxygen gas to find the mass of 0.108 moles of the gas:
\(0.108mol\cdot\frac{32g}{1mol}=3.447g\)5 liters of O2 at 300K and 400torr will weigh 3.447154g.
When a sample of a gas is heated in a sealed, rigid container from 200 K to 400 K, the pressure exerted by the gas is:a) decreased by a factor of 2.b) increased by a factor of 2.c) decreased by a factor of 200.d) increased by a factor of 200.
Answers
the pressure will be increased by a factor of 2.
what is the relationship between the pressure, volume and the temperature ?
The relationship between the pressure, volume, and temperature of a gas is described by the ideal gas law, which is expressed as PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the absolute temperature. According to this law, the pressure of a gas is directly proportional to its temperature, assuming that the volume and number of moles remain constant.
In the given scenario, the gas is heated from 200 K to 400 K, while the container remains sealed and rigid, which means that the volume and number of moles of gas remain constant. As a result, the pressure of the gas must increase by a factor of 2, since the temperature has also increased by a factor of 2.
This can also be explained by the kinetic theory of gases, which states that the pressure of a gas is related to the average kinetic energy of its particles. When the gas is heated, its particles gain kinetic energy and move faster, which causes them to collide with the walls of the container more frequently and with greater force. This increase in collision frequency and force results in an increase in pressure, as described by the ideal gas law.
To learn more about pressure follow the given link: https://brainly.com/question/28012687
#SPJ1
3) A car traveling at a constant velocity of 35 km/h North comes to a full stop 15 seconds
after the driver applies the brakes. What is the acceleration of the car?
A. 2.3 m/s 2
B. -2.3 m/s 2
C. -0.43 m/s 2
D. 0.43 m/s 2

Answers
The acceleration of the car is -2.3m/s².
Explain what an acceleration is.Acceleration is the rate at which the direction and speed of motion change over time. It is said to have been accelerated when something changes its direction and moves faster or slower. Motion on a circle accelerates even when the speed is constant because the direction is constantly changing.
Velocity is the rate at which displacement changes. The rate at which speed changes is known as acceleration. Because it consists of both magnitude and direction, velocity is a vector quantity. Since acceleration is merely the rate at which velocity changes, it too is a vector quantity.
v = u +at
0 = 35 + a×15
15a = -35
a = -35/15
a = -2.3m/s².
To know more about acceleration visit:
https://brainly.com/question/12550364
#SPJ1
Answer:
B. -2.3 m/s²
Explanation:
To answer this question, we have to use the following formula:
\(\boxed{a = \frac{v - u}{t}}\),
where:
• a ⇒ acceleration
• v ⇒ final velocity
• u ⇒ initial velocity
• t ⇒ time taken for the change in velocity to occur
From the question, we know that initially, the car was travelling at 35 km/h. Therefore, u = 35 km/h. The question also tells us that the car comes to a full stop, meaning its final velocity is 0 m/s. Therefore, v = 0 km/h. It takes the car 15 seconds to stop, so t = 15 s.
Using the information above and substituting it into the formula, we can calculate the acceleration of the car:
\(a = \frac{0 - 35}{15}\)
= -2.3 m/s²
Therefore, the acceleration of the car is -2.3 m/s², and the correct answer is B.
2. In which state of matter do particles vibrate in place? *
1 point
A. gas
B. solid
C. liquid
Answers
solid.................
Answer:
b solid
Explanation:
i know for a fact
A solution with a pH of 13.2
would be considered
(A) Strongly acidic
(B) Weakly acidic
(C) Weakly basic
(D) Strongly basic
Answers
Answer:
Strongly Basic
Pls mark Brainliest ;)
In which case are the white balls the maximum distance, and the maximum angle apart?
Answers
The maximum distance between the two white balls is 2R, and the maximum angle between them is 180 degrees (or π radians).
How to solveMaximum distance between the balls:
The maximum distance between the two white balls will be achieved when they are placed at opposite ends of a diameter of the circular region.
In this case, the distance between them will be equal to the diameter of the circle, which is 2R.
Maximum angle between the balls:
To find the maximum angle between the two balls, imagine the center of the circle as the vertex of the angle, and the positions of the two balls as the endpoints of the angle's two sides.
Since the balls are located at opposite ends of a diameter, the angle formed will be a straight angle, which is 180 degrees (or π radians).
So, the maximum distance between the two white balls is 2R, and the maximum angle between them is 180 degrees (or π radians).
Read more about max distance here:
https://brainly.com/question/2264671
#SPJ1
Two white balls, A and B, are placed on a flat surface inside a circular region of radius R. What is the maximum distance and maximum angle between the balls that can be achieved
When a chemical reaction occurs blank happens
Answers
Answer:
In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
Explanation:
What is enthalpy?
A. Enthalpy is the heat involved in a reaction.
B. Enthalpy is the temperature of a reaction.
C. Enthalpy is the mass involved in a reaction.
D. Enthalpy is the kinetic energy of a system.
Answers
Enthalpy is the heat involved in a reaction.
The enthalpy change is the sum of the internal energy and the product of volume and pressure. Enthalpy is the heat involved in a reaction. The correct option is A.
What is enthalpy?The enthalpy denotes the measurement of energy in a thermodynamic system. The amount of enthalpy equals to the total heat content of a system, equivalent to the system's internal energy plus the product of volume and pressure.
The enthalpy is described as a state function, which indicates that its value depends only on the state of the system and it is independent of the path by which this state has been reached.
The equation of the enthalpy of system is given as:
H = U + PV
Here the enthalpy is represented by the letter 'H'. It is a very important quantity as it gives how much heat or energy is present in the system.
Thus the correct option is A.
To know more about enthalpy, visit;
https://brainly.com/question/1657608
#SPJ7
The two main types of weathering are
a
mechanical and physical
b
physical and kinetic
c
chemical and physical
d
chemical and acidic
Answers
Answer:
c. chemical and physical
I hope that’s right :)
Which type of molecule is acetone?
A. Amine
B. Ketone
C. Alcohol
D. Aldehyde
Answers
Answer: B. Ketone
Explanation: Another name for acetone is propanone, and it is the main ketone as it has the functional group in the middle, a CH3 on each side. Ketones have the C=O functional group.