A car traveled 150 meters in 5 seconds, what was its speed? 1.
Pepper the dog traveled 100 meters in 10 seconds, what was Pepper’s speed? 2.
Tony drove his car 1000 meters in 2 minutes, what was the speed of the car? 3.
A bird is flying over New York at a speed of 10 miles per minute. It traveled at this speed for 20 minutes. How far did it fly? 4.
Answer Them all and get 25 points!!!!
Answers
Answer:
1. car traveled 30 meters per second
2. pepper traveled 10 meters per second
3. tony drove around 500 meters per minute
4. the bird traveled 200 miles
Hope this helped!
2.10
3.500
4.200
Related Questions
Vitamin B12, cyanocobalamin, is essential for human nutrition.
Its molecular formula is \(C_{63}H_{88}CoN_{14}O_{14}P\). A lack of this vitamin in the diet can lead to anemia. Cyanocobalamin is the form of the vitamin found in vitamin supplements.
What is the mass (in grams) of \(1.0 * 10^{14}\\\) molecules of cyanocobalamin?
Answers
The mass of 1.0 × 10¹⁴ molecules of cyanocobalamin is equal to 225.03 × 10⁻⁹ g.
What is Avogadro's number?Avogadro’s number is useful to demonstrate the number of entities in one mole of any substance. Generally, these entities can be molecules, electrons, atoms, ions, or protons, which depends on the kind of chemical reaction or reactant and product.
Avogadro’s number is found approximately equal to 6.022 × 10²³ mol⁻¹.
Given, the number of molecules of the cyanocobalamin = 1.0 × 10¹⁴
Given the molecular formula of the cyanocobalamin is C₆₆H₈₈CoN₁₄O₁₄P
The molecular mass of the cyanocobalamin = 1355.38 g/mol
So 6.022 × 10²³ molecules of cyanocobalamin = 1355.38 g
1.0 × 10¹⁴ molecules of cyanocobalamin= 1355.38 ×(1.0× 10¹⁴/6.022×10²³)
= 225.03 × 10⁻⁹ g
Learn more about Avogadro's number, here:
brainly.com/question/11907018
#SPJ1
A change in tempo is the only thing that can make a musical cliché more effective.
A. False, a change in dynamics is the only thing that can make a musical cliché more effective.
B. True
C. False, using changes in tempo and dynamics can make a musical cliché more effective.
D. False, a change in tempo cannot make a musical cliché more effective.
Answers
False, using changes in tempo and dynamics can make a musical cliché more effective. Hence, option C is correct.
What is musical tempo ?Tempo in a music refers to pace of the music especially in high pitch sound waves. The most common unit of measurement for tempo is beats per minute (BPM), which is just the quantity of beats that may be captured in a minute at a certain pace.
A musical segment or composition is slower when the beats per minute is lower than when the BPM is greater. While a higher BPM is more likely to make the listener feel excited, a lower BPM is more likely to have a calming and/or sad effect on the listener.
Changing dynamics is some more effective way to make musical cliché. Hence, option C is correct.
Find more on musical tempo:
https://brainly.com/question/29574317
#SPJ1
A student made a sketch of a potential energy diagram to represent an endothermic reaction.
A curve line graph is shown. The y axis of the graph has the title Potential Energy and kJ written in parenthesis. The x axis of the graph has the title Reaction Pathway. The curve begins at a higher level and ends at a slightly lower level. A broken horizontal line is shown from a point labelled X on the y axis to the point where the curve begins. Another broken horizontal line is shown from a point labeled Y on the y axis to the point where the curve ends.
Explain, using complete sentences, why the diagram made by the student is correct or incorrect. Be sure to also explain what the values of X and Y represent.
Answers
Based on the description of the potential energy diagram provided, the diagram made by the student appears to be correct.
The potential energy diagram represents the energy changes that occur during a chemical reaction. In an endothermic reaction, the products have higher potential energy than the reactants, meaning energy is absorbed from the surroundings.
The curve line on the graph indicates the energy changes throughout the reaction pathway. It starts at a higher level, representing the initial potential energy of the reactants. As the reaction progresses, the potential energy decreases, indicating the formation of products with lower potential energy.
The broken horizontal line from point X on the y-axis to the point where the curve begins represents the activation energy (Ea) of the reaction. Activation energy is the energy barrier that must be overcome for the reactants to convert into products.
Point X on the y-axis indicates the potential energy of the reactants at the start of the reaction, and the broken line shows the energy required to initiate the reaction.
The broken horizontal line from point Y on the y-axis to the point where the curve ends represents the potential energy of the products. Point Y represents the potential energy of the products at the end of the reaction.
Overall, the student's diagram correctly represents an endothermic reaction, showing the potential energy changes, the activation energy, and the final potential energy of the products. The curve line starts at a higher level (representing the higher potential energy of the reactants) and ends at a slightly lower level (representing the lower potential energy of the products).
For more such questions on potential energy diagram visit;
https://brainly.com/question/23343697
#SPJ8
water has low ____ _____
Answers
Water has low viscosity and low surface tension.
Viscosity refers to the resistance of a fluid to flow, and water has a relatively low viscosity compared to many other liquids. This property is important for many applications, including the movement of water through pipes and the flow of water in rivers and streams.
Surface tension, on the other hand, is the measure of the cohesive force between molecules at the surface of a liquid. Water has a relatively low surface tension compared to some other liquids, which makes it easier for objects to penetrate its surface. This property is also responsible for the formation of droplets and the behavior of water striders and other water-dwelling insects.
Overall, these properties contribute to the unique characteristics of water and its importance in many natural and industrial processes.
For more such questions on viscosity
https://brainly.com/question/17712969
#SPJ11
Polar water molecules can surround ions, reducing the likelihood of them interacting with other ions. What property of water does this phenomenon cause?
Answers
The property of water that causes polar water molecules to surround ions, reducing the likelihood of them interacting with other ions, is known as its solvation or hydration ability.
This solvation property is a result of water's high polarity, which arises from its asymmetrical molecular structure and the presence of polar covalent bonds. Water molecules have a partially positive (+) and partially negative (-) end due to the electronegativity difference between oxygen and hydrogen atoms. This polarity enables water molecules to form hydrogen bonds with other water molecules and with polar solutes, such as ions. When ions dissolve in water, the partially positive hydrogen atoms of water molecules are attracted to the negatively charged ions, and the partially negative oxygen atoms of water molecules are attracted to the positively charged ions.
Learn more about the hydrating nature of water here.
https://brainly.com/question/2709403
#SPJ1
The pH at the equivalence point of a titration may differ from 7.0 due to:_______.
Answers
Answer:
hydrolysis of the resulting salt
Explanation:
The pH at the equivalence point of a titration is not always 7.0 due to the hydrolysis of resulting salt.
The pH at the equivalence point of a titration involving a strong acid and a strong base will be 7 because the conjugate base of the salt formed will be too weak to react with water. For example, the titration of HCl and NaOH:
\(HCl + NaOH --> NaCl + H_2O\)
In the case of strong acid and weak base, the pH at the equivalence point will be acidic because the salt formed would dissociate and react with water. For example, the titration of HCl with NH3:
\(NH_3+HCl-->NH_4Cl\)
The resulting salt dissociates in water and releases proton to form an acidic endpoint.
\(NH_4^{+} + H_2O --> H_3O^+ + NH_3\)
For weak acid and strong base, the pH at the equivalence point will be alkaline because the resulting salt dissociates in water to form an alkaline solution.
\(NaOH + CH_3COOH --> CH_3COONa + H_2O\)
\(CH_3COO^- + H_2O --> CH_3COOH + OH^-\)
Which process do self-feeders use to get energy?

Answers
Answer:
The person above me is correct
Explanation:
It's D
4. How many moles of oxygen gas are needed to react completely with
3.0 moles of C2,H6.?
Answers
Hey there!:
Mole ratio :
2 C₂H₆ + 7 O₂ → 4 CO₂ + 6 H₂O
2 moles C₂H₆ -------------------- 7 moles O₂
3.0 moles C₂H₆ ------------------ moles O₂ ??
moles O₂ = 3.0 x 7 / 2
moles O₂ = 21 / 2
moles O₂ = 10.5 moles of O₂
Hope this helps!
3. Write the following isotope in hyphenated form (e.g., "carbon-14”): Kr
a. Krypton-109
b. Krypton -37
c. Krypton -36
d. Krypton -73
Answers
Answer:
Krypton -73
Explanation:
There are 33 known isotopes of krypton (36Kr) with atomic mass numbers from 69 through 101.
Good luck!
Answer:
D. Krypton-73
Explanation:
An isotope of an element has the same atomic number and the same number of protons but a different number of neutrons and a different atomic weight. Krypton is the 36th element on the periodic table. It has 36 protons and 48 neutrons. Krypton-73 is one of 33 known isotopes of Krypton and is the only one that actually exists from the list of choices.
Hope that helps.
CuI2 (light brown solid) name copper compounds
Answers
CuI2 is not a known compound. Copper compounds typically have different oxidation states for copper, resulting in various compound names.
Copper(II) oxide (CuO): It is a black solid compound where copper is in the +2 oxidation state. It is commonly used as a pigment and in catalytic reactions.
Copper(II) sulfate (CuSO4): It is a blue crystalline compound in which copper is in the +2 oxidation state. It is used in various applications such as agriculture, electroplating, and as a laboratory reagent.
Copper(I) oxide (Cu2O): It is a red crystalline compound in which copper is in the +1 oxidation state. It is used as a pigment, in solar cells, and as a catalyst.
Copper(II) chloride (CuCl2): It is a greenish-brown solid compound in which copper is in the +2 oxidation state. It is utilized in various chemical processes, including etching and catalyst synthesis.
Copper(II) nitrate (Cu(NO3)2): It is a blue crystalline compound where copper is in the +2 oxidation state. It is commonly used in the production of catalysts, as a coloring agent, and in electroplating.
These are just a few examples of copper compounds with different oxidation states and properties. It's important to note that the compound CuI2 mentioned in the question, if it exists, would be an exception to the typical nomenclature for copper compounds.
For more such questions on oxidation visit:
https://brainly.com/question/13182308
#SPJ8
a 1. The electron configuration a particular metal contena de is [Ar]3d a) Identify the Corresponding metal b) write the electron configuration 3 the metal ion
Answers
Explanation:
if it was meant for [Ar]4s²3d¹ :
1s²2s²2p⁶3s²3p⁶4s²3d¹
number of e‐ : 21
atomic number is 21.. element is scandium
ion: Sc³+
If you can answer it with another sheet answering all that I would appreciate it.

Answers
Boyle's law is a fundamental principle in physics that describes the relationship between the pressure and volume of a gas, when temperature and the number of particles are kept constant.
What is Boyle's law?It states that the pressure of a gas is inversely proportional to its volume, meaning that if the volume of a gas decreases, its pressure will increase, and if the volume of a gas increases, its pressure will decrease.
1)
P1V1 = P2V2
P2 = P1V1/V2
P2 = 1 * 1000/473
P2 = 2.1 atm
2)
V2 = P1V1/P2
V2 = 1 * 2/6 * 10^4
V2 = 3.3 * 10^-5 L
3) V2 = P1V1/P2
V2 = 2 * 10^6 * 1 * 10^-5/0.275
V2 = 72.7 L
4)
V2 = P1V1/P2
V2 = 3.04 * 10^4 * 10/150
V2 = 2027 L
Learn more about Boyle's law:https://brainly.com/question/30367067
#SPJ1
what mass of TiCl4 must react with an excess of water to produce 50.0g of TiO2 if the reaction has a 78.9% yield
Answers
Answer:
\large \boxed{\text{150 g TiCl}_{4}}
Explanation:
We will need a balanced chemical equation with masses and molar masses, so, let's gather all the information in one place.
Mᵣ: 189.68 79.87
TiCl₄ + 2H₂O ⟶ TiO₂ + 4HCl
m/g: 50.0
To solve this stoichiometry problem, you must
Convert the actual yield to the theoretical yield Use the molar mass of TiO₂ to convert the theoretical yield of TiO₂ to moles of TiO₂ Use the molar ratio to convert moles of TiO₂ to moles of TiCl₄ Use the molar mass of TiCl₄ to convert moles of TiCl₄ to mass of TiCt₄1. Theoretical yield of TiO₂
\(\text{Theoretical yield} = \text{50.0 g actual} \times \dfrac{\text{100 g theoretical}}{\text{78.9 g actual}} = \text{63.37 g theoretical}\)
2. Moles of TiO₂
\(\text{Mass of TiO}_{2} = \text{63.37 g TiO}_{2} \times \dfrac{\text{1 mol TiO}_{2}}{\text{79.87 g TiO}_{2} } = \text{0.7934 mol TiO}_{2}\)
3, Moles of TiCl₄
The molar ratio is 1 mol TiO₂:1 mol TiCl₄.
\(\text{Moles of TiCl}_{4} = \text{0.7934 mol TiO}_{2} \times \dfrac{\text{1 mol TiCl}_{4}}{\text{1 mol TiO}_{2}} = \text{0.7934 mol TiCl}_{4}\)
4. Mass of TiCl₄
\(\text{Mass of TiCl}_{4} = \text{0.7934 mol TiCl}_{4} \times \dfrac{\text{189.98 g TiCl}_{4}}{\text{1 mol TiCl}_{4}} =\textbf{150 g TiCl}_{\mathbf{4}} \\\\\text{You must use $\large \boxed{\textbf{150 g TiCl}_{\mathbf{4}}}$}\)
Identify the following reaction: 2Al + 3CuCh + 2AICh + 30u
Answers
Answer:
The chemical reaction identified is:
2Al + 3CuCl2 → 2AlCl3 + 3Cu
In this reaction, aluminum (Al) reacts with copper chloride (CuCl2) to form aluminum chloride (AlCl3) and copper (Cu). The coefficients in front of the chemical formulas indicate the stoichiometric ratio of the reactants and products, which means that two moles of Al react with three moles of CuCl2 to produce two moles of AlCl3 and three moles of Cu.
Washing soda crystals react with acid to give off carbon dioxide. If you added some washing soda crystals to vinegar, what would you see happening
Answers
The reaction can be represented by the following chemical equation:
Na2CO3 + 2CH3COOH → 2CO2 + 2H2O + 2NaCH3COO
As the reaction proceeds, bubbles of carbon dioxide gas will be released, which will cause the solution to fizz and bubble. The solution may also become warmer due to the exothermic nature of the reaction. The resulting solution will be a mixture of sodium acetate and water.
It's worth noting that the reaction will only occur if there is enough vinegar to react with the washing soda crystals. If there is an excess of washing soda crystals, some of them may not react and will remain in the solution. Similarly, if there is an excess of vinegar, some of it may remain unreacted.
What is the osmotic pressure formed by dissolving 44.2 mg
of aspirin (C9H8O4) in 0.358 L of water at 25 °C?
Answers
By dissolving 44.2 mg of aspirin in 0.358 L of water at 25 °C, an osmotic pressure of 0.00578 atm is created.
What is osmotic pressure?When aspirin is dissolved in water, osmotic pressure ( π ) = iMRT
Where,
π = The osmotic pressure is (in atmospheres)i = The van't Hoff factor, or I measures how many particles a solute in solution can separate into (in this case, aspirin does not separate in water, hence I = 1).M = solution's molarity (in moles per liter)R = gas constant, or R, is 0.08206 L atm/(mol K).T =he absolute temperature is T. (in Kelvin)What is molar mass?Molar mass of aspirin (C9H8O4) = 180.16 g/mol
44.2 mg = 0.0442 g
Moles of aspirin = 0.0442 g / 180.16 g/mol
= 0.000245 moles
Molarity of aspirin solution
= moles / volume = 0.000245 moles / 0.358 L
= 0.000685 M
π = iMRT {on equating both the equations}
= (1)(0.000685 M)(0.08206 L·atm/(mol·K))(25 + 273.15 K)
= 0.00578 atm
To know more about Osmotic pressure
https://brainly.com/question/12497098
#SPJ1
The energy stored in an object is called potential energy
True or false
Answers
its true
Potential energy is the stored or latent energy in an object at rest. It’s fundamental to many physics-related concepts because its laws hold true on any level, from the planetary to the atomic level. The potential energy of an object is measurable.
A 200 N force is applied to an object, which then accelerates at 2 m/s². What is the mass of the object?
Answers
The mass of the object that is acted on by a force of 200 N is 100 kg
What is mass?Mass can be defined as the quantity of matter a body contains.
To calculate the mass of the object, we use the formula below.
Formula:
m = F/a................ Equation 1Where:
m = Mass of the objectF = Force of the objecta = Acceleration due to gravityFrom the question,
F = 200 Na = 2 m/s²Substitute these values into equation 1
m = 200/2m = 100 kgHence, the mass of the object is 100 kg.
Learn more about mass here: https://brainly.com/question/19385703
#SPJ1
Which can conduct heat faster metal or air ( gas)? Explain your answer.
Answers
Metals conduct heat faster than air. Air or gas heats up by way of convection, whereas metals are closely packed with one another.
Which can conduct heat faster metal or air gas?substances that are poor conductors of thermal energy are called thermal insulators. Gases such as air and matter such as plastic Metal and wood are thermal insulators.
Metals and stones are examined as good conductors since they can rapidly transfer heat, whereas materials like wood, paper, air, and cloth are the poor maestros of heat. In particular, metals conduct heat better than non-metals, and solids conduct heat better than gases.
So we can conclude that All metals 'can' flatter a gas. If you heat them in a vacuum, all elemental metals can become a gas
Learn more about metal here: https://brainly.com/question/4701542
#SPJ1
When the volume of a gas is changed from 11.5 cm3 to __ cm3, the temperature will change from 415.0 K to 200.0 K

Answers
Answer:
Explanation:
Charles Law: The volume of and ideal gas is directly proportional to the temperature when the pressure is kept constant
V₁ - initial Volume = 11.5 cm³ ; T₁ = Initial temperature = 415 K ;
V₂ - final volume ;T₂ =final temperature = 200 K
\(\dfrac{V_{1}}{T_{1}}=\dfrac{V_{2}}{T_{2}}\\\\\\\dfrac{11.5}{415}=\dfrac{V_{2}}{200}\\\\\\\dfrac{11.5}{415}*200=V_{2}\\\\\\V_{2}=5.5 \ cm^{3}\)
WILL GIVE BRAINEST + 500 PTS ...
1.Scientists have evidence that the universe is [expanding/shrinking].
2.Objects moving away from Earth appear [blue/red], and objects moving toward Earth appear [blue/red].
3. Light from most other galaxies appears [blue/red], which makes sense if the universe is [collapsing inward/spreading out].
Answers
Answer:
1. expanding
2. red, blue
3. red, spreading out
Explanation:
Cyanide gas has a pressure of 6.0atm.If the pressure is decreased to 0.5atm and the final volume is 48L, what was the original volume?
Answers
ANSWER
The original volume of the gas is 4L
EXPLANATION
Given information
The initial pressure of the gas = 6.0atm
The final pressure of the gas = 0.5 atm
The final volume of the gas = 48L
Step 1: Write the Boyle's law formula
\(\text{ P1}\times V1\text{ = P2}\times V2\)Where
P1 = 6.0 atm
P2 = 0.5 atm
V2 = 48L
V1 =?
Step 2: Substitute the given data into the formula in step 1
\(\begin{gathered} 6.0\times V1\text{ = 0.5}\times\text{ 48} \\ 6.0\text{ }\times\text{ V1 = 24} \\ \text{ Divide both sides by 6} \\ \text{ V1 = }\frac{24}{6} \\ \text{ V1 = 4L} \end{gathered}\)Hence, the original volume of the gas is 4L
Which of the following statements involving the periodic table is not correct?
A. The transition metals are in Groups 3 through 12 of the periodic table.
B. Uranium (Z = 92) is in the seventh period of the periodic table.
C. Alkali metal ions always have a charge of +1.
D. The element most similar to oxygen in its chemical behavior is fluorine.
E. The charge on monatomic halogen ions in binary ionic compounds is –1.
F. Silicon is a semimetal (metalloid).
Answers
Answer: D
Explanation:
oxygen is in different group with fluorine hence is not the most similar they might have similalities but is not the most similar
identify the description that applies to the substance represented by each nfpa diamond. an nfpa diamond is made of 4 smaller diamonds: blue on the left with a 3 in it, red on top with a 0 in it, yellow on the right with a 0 in it, and white on the bottom with o x in it. oxidizer, not flammable an nfpa diamond is made of 4 smaller diamonds: blue on the left with a 3 in it, red on top with a 0 in it, yellow on the right with a 2 in it, and white on the bottom. choose... an nfpa diamond is made of 4 smaller diamonds: blue on the left with a 2 in it, red on top with a 4 in it, yellow on the right with a 0 in it, and white on the bottom with c o r in it. choose... an nfpa diamond is made of 4 smaller diamonds: blue on the left with a 2 in it, red on top with a 3 in it, yellow on the right with a 0 in it, and white on the bottom.
Answers
Oxidizer 3-0-0-oxy is not flammable. 3-0-2 is reactive or moderately unstable. The flash point of 2-4-0 is below the ambient temperature. Health risk are there but no specific dangers are in 2-3-0 stable compound.
A nfpa diamond that is made up of 4 smaller diamonds: blue on the left with a 3 in it, red on top with a 0 in it, yellow on the right with a 0 in it, and white on the bottom with o x in it is Oxidizer 3-0-0-oxy i.e., not flammable.
A nfpa diamond that is made up of 4 smaller diamonds: blue on the left with a 3 in it, red on top with a 0 in it, yellow on the right with a 2 in it, and white on the bottom is - 3-0-2 i.e., reactive or moderately unstable.
A nfpa diamond that is made up of 4 smaller diamonds: blue on the left with a 2 in it, red on top with a 4 in it, yellow on the right with a 0 in it, and white on the bottom with c o r in it indicates that the flash point of 2-4-0 is below the ambient temperature.
A nfpa diamond that is made up of 4 smaller diamonds: blue on the left with a 2 in it, red on top with a 3 in it, yellow on the right with a 0 in it, and white on the bottom results in health risk but no specific dangers are there in 2-3-0 stable compound.
Here is more information about health risk : brainly.com/question/2122909
#SPJ4
Each of the following reactions were in equilibrium when the pressure of their containers was doubled. Chose which way the reaction shifted after the pressure change:
2NH3 (g)㈠No(g) +3H2(g) [Select ]
2Na3 PO4 (aq) + 3CaCl2 (aq) ㈠ Ca3 (PO4 )2 (s) + 6NaCl(aq) [Select ]
2C0(g)+O2 (g)->2CO2 (g) [Select ]
2H1(g) ㈠ H2(g) + 12(g) Neither
Answers
Answer:
Each of the following reactions was in equilibrium when the pressure of their containers was doubled. Chose which way the reaction shifted after the pressure change:
2NH3 (g)->N2(g) +3H2(g)
2Na3 PO4 (aq) + 3CaCl2 (aq) -> Ca3 (PO4 )2 (s) + 6NaCl(aq)
2CO(g)+O2 (g)->2CO2 (g)
2HI(g) -> H2(g) + I2(g)
Explanation:
Effect of pressure on equilibrium:
When pressure is increased on an equilibrium system,then equilibrium will shift in such a direction towards less number of moles of substrates.
For the first system,
2NH3 (g)->N2(g) +3H2(g)
increase in pressure,shifts the equilibrium towards the left side that is the formation of ammonia is favored.
For the second reaction:
2Na3 PO4 (aq) + 3CaCl2 (aq) -> Ca3 (PO4 )2 (s) + 6NaCl(aq)
The quilibrium will shift towards right.
Becuase right side less number of moles of substrates are there.
2CO(g)+O2 (g)->2CO2 (g)
For this system,the equilibrium will shift towards right side that is formation of CO2 gas is favored.
For the last system,
2HI(g) -> H2(g) + I2(g)
there is no effect of pressure.
Becuase the number of moles of substrates are same on both sides.
a. Phenol (C6H5OH) is an aromatic compound and a colourless liquid widely used in household products
and medicine. When it reacts with chlorine liquid (chlorine is a diatomic molecule), in the presence of a
catalyst, one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine
atom, and liquid chlorophenol is formed. This replacement process is called chlorination
Write a balanced chemical equation for the chlorination reaction and explain how you balanced it. Note
that hydrogen chloride gas (HCI) is also a product of this chemical reaction and you should ignore the
presence of the catalyst in the equation.
Answers
Because there are 2 Cl on the left, we will put a coefficient 2in front of HCl on the right side to balance out the Cl. This would result in an unequal amount of H, with 6 on the right side and 7 in the left, so we have to put a coefficient of 2 in front of C6H5OH and C6H4OH on both sides to balance out the H. By doing this, we would obtain an equal amount of H on both sides. The Carbon is already balanced, and so is the Oxygen.
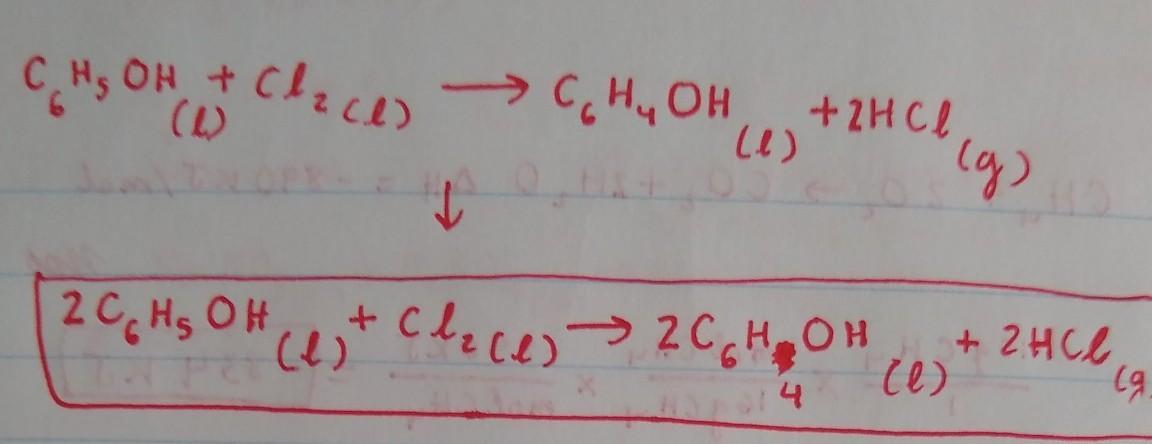
The balanced chemical reaction equation for the reaction between aromatic phenol and chlorine gas in the presence of FeCl3 as catalyst is as follows;
C6H6O + Cl2 -------> C6H5OCl + HCl
An aromatic compound has 4n + 2 number of pi electrons. This condition is satisfied by phenol. Hence, phenol has the stability associated with aromatic compounds.
The reaction of phenol with chlorine in the presence of a catalyst such as FeCl3 is an aromatic electrophillic substitution reaction.
This reaction yields a chlorinated phenol (one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine atom and chlorophenol is formed).
A balanced chemical reaction equation is one in which the number of atoms of each element at the reactant side and the product side are equal. This condition is satisfied for the reaction;
C6H6O + Cl2 -------> C6H5OCl + HCl
Learn more; https://brainly.com/question/6170291
what is the kg of the net force is 30 N and the acceleration is 6 m/s 2
Answers
Answer:
5kg
Explanation:
Use the law: F=MA
F= force which is newton
M= mass which is kg
A= acceleration which is \(m/s^{2}\)
I need help with this

Answers
The theoretical yield of water formed from the reaction of 46.8g of octane and 287g of oxygen gas is 58.58 g.
What is a balanced chemical equation?A balanced chemical equation is a representation of a chemical reaction using chemical formulas and symbols. It shows the reactants and products of the reaction, and the coefficients in front of the formulas ensure that the number of atoms of each element is the same on both sides of the equation.
The balanced chemical equation for the reaction between octane and oxygen gas is:
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
According to the stoichiometry of the balanced equation, 2 moles of octane react with 25 moles of oxygen gas to produce 18 moles of water. Therefore, the molar ratio of octane to water is 2:18 or 1:9.
First, we need to determine which reactant is limiting, i.e., which reactant will be completely consumed in the reaction. To do this, we can calculate the amount of water that would be produced if each reactant were to react completely and then compare the results.
The molar mass of octane (C8H18) is 114.23 g/mol, so 46.8 g of octane is equal to:
46.8 g / 114.23 g/mol = 0.41 mol
The molar mass of oxygen gas (O2) is 32.00 g/mol, so 287 g of oxygen gas is equal to:
287 g / 32.00 g/mol = 8.97 mol
Now, we can calculate the theoretical yield of water based on the amount of each reactant:
Octane: 0.41 mol octane × 9 mol H2O/1 mol octane = 3.69 mol H2O
Oxygen gas: 8.97 mol oxygen gas × 9 mol H2O/25 mol O2 = 3.25 mol H2O
Since the stoichiometry of the reaction indicates that the amount of water produced should be proportional to the amount of octane used, and the calculation shows that less water would be produced if we used all of the oxygen gas, we conclude that oxygen is the limiting reactant.
Therefore, the theoretical yield of water formed is 3.25 mol H2O × 18.02 g/mol = 58.58 g H2O.
To know more about balanced chemical equation, visit:
https://brainly.com/question/28294176
#SPJ1
A 19.48 gram sample of copper is heated in the presence of excess oxygen. A metal oxide is formed with a mass of 21.94 g. Determine the empirical formula of the metal oxide.
Answers
A 19.48 gram sample of copper is heated in the presence of excess oxygen. A metal oxide is formed with a mass of 21.94 g. Then the empirical formula of the metal oxide is Cu₃O.
The mass of Cu is 19.48 grams.
The mass of the metal oxide is 21.94 grams.
The mass of oxygen is (21.94 – 19.48) = 2.46 grams
The atomic mass of Cu = 63.546
The atomic mass of O = 15.9
So:
Moles of Cu = 19.48/63.546 = 0.3 moles
Moles of O = 2.46/21.94 = 0.1 mol
Mol Cu : Mol O = 0.3 : 0.1 = 3 : 1
So, the empirical formula is Cu₃O
Learn more about empirical formulas at https://brainly.com/question/13058832.
#SPJ1
Describe an
application for the water collecting surface (where could it be used and for
what purpose?)
Answers
Answer:
Rainwater harvesting system, also called rainwater collection system or rainwater catchment system, technology that collects and stores rainwater for human use. Rainwater harvesting systems range from simple rain barrels to more elaborate structures with pumps, tanks, and purification systems. The nonpotable water can be used to irrigate landscaping, flush toilets, wash cars, or launder clothes, and it can even be purified for human consumption. With water scarcity a pressing problem for many densely populated regions, rainwater harvesting systems can supply households and businesses with water for use in dry seasons and lessen the demand on municipal systems.
Explanation: