a chemical bond formed when two atoms share four electrons is a bond; it is best described as .
Answers
A chemical bond formed when two atoms share four electrons is a double bond. It is best described as a covalent bond.
A double bond is a bond which is formed when two atoms share four electrons between them. When such bonds are formed by sharing of atoms, it is known as a covalent bond. The electron pair in the atom, which makes covalent bonds, are known as bonding pairs. When electron pairs are shared, the outermost shell of the atom achieves stability, which is similar to noble gases. When two electrons are shared by two atoms, it is known as a single covalent bond. When six electrons are shared by two atoms, it is known as a triple covalent bond.
Therefore, a chemical bond formed when two atoms share four electrons is a double bond, and it is best described as a covalent bond.
To know more about covalent bond
https://brainly.com/question/9140585
#SPJ1
Related Questions
Fatty acid groups are referred to as ________ groups.
A) Acetyl
B) Acyl
C) Prenyl
D) Isoprenoid
E) Isopentenyl
Answers
Fatty acid groups are referred to as B) acyl groups.
Fatty acids are organic compounds that consist of a long hydrocarbon chain with a carboxyl group (-COOH) at one end. The hydrocarbon chain is composed of carbon and hydrogen atoms, and its length can vary. Fatty acids play essential roles in various biological processes and are major components of lipids, including triglycerides and phospholipids.
When a fatty acid is involved in chemical reactions or is attached to other molecules, it typically undergoes a process called activation, where it is converted into an acyl group. An acyl group is formed by replacing the -OH (hydroxyl) group of the carboxyl group with an -OR (alkoxy) group. The -OR group can be derived from various molecules, such as coenzyme A (CoA) or other acyl carrier proteins.
For example, when a fatty acid is activated for incorporation into a triglyceride molecule, it forms a triglyceride acyl group. Similarly, when a fatty acid is incorporated into a phospholipid molecule, it forms a phospholipid acyl group. The acyl group represents the hydrocarbon chain of the fatty acid, which may vary in length and saturation.
Therefore, the correct answer to the question is B) Acyl.
Learn more about Fatty acids https://brainly.com/question/27960389
#SPJ11
how many atoms in al(c2h3o2)3
Answers
Answer:
1 + (2+3+2)×3
1+7×3
1+21
22
Explanation:
please mark me as brainliest
Answer: 1 + 6 + 9 + 6 = 22
Explanation:
al = 1
c = 6
h = 9
o = 6
Atoms tend to form compounds because they have an unstable number of ___
A. Protons
B. Isotopes
C. Valence electrons
D.neutrons
Answers
I have 130 L of gas in a piston at a temperature of 250°C. If I cool the gas
until the volume decreases to 85 L, what will the temperature of the gas be?
Answers
This is a Charles' Law problem: V1/T1 = V2/T2. As the temperature of a fixed mass of gas decreases at a constant pressure, the volume of the gas should also decrease proportionally. Here, we are given the new volume of the gas after cooling, and we want to determine to what temperature the gas was cooled.
To use Charles' Law, the temperature must be in Kelvin (x °C = x + 273.15 K). We want to solve Charles' Law for T2, which we can obtain by rearranging the equation into T2 = V2T1/V1. Given V1 = 130 L, T1 = 250 °C (523.15 K), and V2 = 85 L:
T2 = (85 L)(523.15 K)/(130 L) = 342 K or 68.9 °C. If sig figs are to be considered, since all the values in the question are given to two sig figs, the answer to two sig figs would be either 340 K or 69 °C.
Gases show great uniformity in their behaviour irrespective of their nature. The concept Charles's law is used here to determine the new temperature of the gas. The new temperature of the gas is 341.96 K.
What is Charles's law?At constant pressure, the volume of a given mass of gas is directly proportional to the temperature on Kelvin scale. The absolute scale of temperature is introduced by the scientist Charles. The temperature at which gases supposed to have zero volume is called the absolute temperature.
Mathematically the Charles's law can be expressed as:
V = constant × T
V / T = constant
For two different gases, the equation is:
V₁ / T₁ = V₂ / T₂
T₂ = V₂T₁ / V₁
85 × 523 / 130 = 341.96 K
Thus the new temperature of the gas is 341.96 K.
To know more about Charles's law, visit;
https://brainly.com/question/16927784
#SPJ2
What is the molecular formula of a compound with a percent composition of 49.47% C, 5.201% H, 28.84% N, and 16.47% O, and a molar mass of 194.2 g/mol?
Answers
Answer:
Explanation:
The molecular formula is the true chemical formula of a covalent molecular compound, and a multiple of the empirical formula. We can use the molar mass, along with the molar empirical mass, determined from the empirical formula, to determine the molecular formula of the compound.
Using, the percentage composition of each element, we can say: In each 100g of the compound, there is 49.47g (C), 5.201g (H), 28.84g (N), and 16.47g (O).
Therefore, we can now find the number of moles of each element, in 100g of compound. number of moles (n) = mass present (m) ÷ molar mass (M). Molar mass can be found using a standard IUPAC Periodic Table.
n(C) = m/M = 49.47/12.01 = 4.1191 mol
n(H) = m/M = 5.201/1.008 = 5.1597 mol
n(N) = m/M = 28.84/14.01 = 2.0585 mol
n(O) = m/M = 16.47/16.00 = 1.0293 mol
Since the empirical formula is one in which the proportions of each element are expressed in the simplest mole ratio, therefore:
C : H : N : O = 4.1191 : 5.1597 : 2.0585 : 1.0293
Divide each ratio by 4.1191 =
1 : 1.00985 : 0.4997 : 0.249, which is approximately equal to:
1 : 1 : 0.5 : 0.25 = 2 : 2 : 1 : 0.5 = 4 : 4 : 2 : 1
Therefore, empirical formula = C₄H₄N₂O.
Using empirical formula, we can calculate molar empirical mass, which is found by summing the molar atomic weights.
molar empirical mass = 4(12.01)+4(1.008) + 2(14.01) + 16.00 = 96.092.
The molar empirical mass is double the molar mass, and therefore, molecular formula must be half the empirical formula.
Therefore, molecular formula = C₂H₂NO
Liquid to Gas =
Solid to Liquid =
Answers
The process of a liquid becoming a gas is called boiling (or vapourization) and the process of a solid becoming a liquid is called melting.
Vaporization is the process of converting a liquid into a gas. It is also called evaporation. Since we know that the particles of a gas are moving faster than those of a liquid, an input of energy must be required for a liquid to become a gas.
Melting is a physical process that results in the phase transition of a substance from a solid to a liquid. During melting, the energy goes exclusively to changing the phase of a substance; it does not go into changing the temperature of a substance. Hence melting is an isothermal process because a substance stays at the same temperature.
Example 1: Industrially, salt is recovered from seawater by the process of vaporization. Wet clothes are dried up due to the process of vaporization. The process is used in many industrial processes for separating the components of a mixture.
Example 2: Ice to water - Ice melts back into the water when it is left out at temperatures above the freezing point of 32 degrees. Rocks to lava - Rocks in volcanoes can be heated until they are molten lava. Metal to molten liquid - Metals such as steel and bronze can be molten down.
To learn more about vaporization and melting visit,
https://brainly.com/question/17945501
which of the following types of radiation can be blocked with only a sheet of paper?
beta decay, gamma decay, they are equally dangerous, alpha decay
Answers
Answer:
Alpha decay
Explanation:
Alpha decay can be blocked with only a sheet of paper.
Extra info : -
In general, alpha particles have a very limited ability to penetrate other materials. In other words, these particles of ionizing radiation can be blocked by a sheet of paper, skin, or even a few inches of air.
Hello there!
Alpha decay can be blocked with only a sheet of paper.Gamma rays can only be blocked with something thick and dense.Beta rays can be blocked with something less dense, but thicker than paper.Hope this helps. Let me know if you have any questions.
~GracefulGirlie :)
Good luck on your assignment.
Organic molecules are defined as molecules that contain.
Answers
Answer:
, organic compounds include all molecules that contain carbon.
Explanation:
carbon
Which images show a container holding a heterogeneous mixture?

Answers
Is oil a reasonable energy source? Why or why not?
Answers
Answer:
No, it is not.
Explanation:
Oil is a non-renewable source of energy. ... Burning oil can pollute the air. Much of our oil has to be imported and it is becoming more and more expensive as reserves reduce and imports increase. Producing electricity from crude oil is expensive compared to other fossil fuels such as coal or gas.
*You Can put this in your own words
For which reaction is ΔG° expected to be closest to ΔH°?
CO2(g) ⇄ CO2(s)
2NO(g) ⇄ N2(g) + O2(g)
H2O(ℓ) ⇄ H2O(s)
NaCl(s) ⇄ Na+(aq) + Cl-(aq)
N2(g) + 3H2(g) ⇄ 2NH3(g)
Answers
The H2O(ℓ) ⇄ H2O(s) response is ΔG° and is expected to be closest to ΔH°.
Option c is correct.
We would expect ΔG° to be closest to ΔH° for the reaction in which the reactant and product states are most similar. Therefore, the reactions in which ΔG° is expected to be closest to ΔH° are those involving a phase change from gas to solid or liquid. This is because they typically involve small changes in entropy (ΔS°).
The third reaction given is H2O(ℓ) ⇄ H2O(s), which involves a phase change. This is a reversible reaction involving melting or freezing of water, and the difference between the standard change in free energy (ΔG°) and the standard change in enthalpy (ΔH°) is expected to be small. Therefore, ΔG° is expected to be the closest to ΔH° for this reaction.
Hence, Option c is correct.
To know more about reversible reaction visit :
https://brainly.com/question/16614705
#SPJ4
The Diffusivity of chloroform in air was measured using an Arnold Cell at 298K and 1 atm. The liquid density of chloroform at 298K is 1.485 g/cm3 and its vapor pressure at 298K is 2.67x104 Pa. Initially, the liquid chloroform surface was 7.4 cm from the top of the tube, and after 10 hours the liquid surface dropped to 7.96 cm. If the concentration of chloroform is zero at the top of the tube, what would be the binary diffusion coefficient of chloroform in air.
Answers
The binary diffusion coefficient of chloroform in air is approximately -6.54 x 10⁻¹² m²/s.
To determine the binary diffusion coefficient of chloroform in air, we can use Fick's Law of Diffusion, which relates the change in concentration with time to the diffusion coefficient and other relevant parameters.
First, we need to calculate the change in concentration of chloroform in the tube over the given time period. This change in concentration can be determined by measuring the change in height of the liquid surface.
Change in concentration = (Initial height - Final height) / Initial height
Initial height = 7.4 cm
Final height = 7.96 cm
Change in concentration = (7.4 cm - 7.96 cm) / 7.4 cm
Next, we need to calculate the average concentration of chloroform in the tube. This can be done by multiplying the change in concentration by the liquid density of chloroform.
Average concentration = Change in concentration × Liquid density
Liquid density of chloroform = 1.485 g/cm³
Average concentration = [(7.4 cm - 7.96 cm) / 7.4 cm] × 1.485 g/cm³
Now, we can use Fick's Law of Diffusion to calculate the diffusion coefficient (D):
Change in concentration / Time = (D × Average concentration) / Distance
Time = 10 hours = 10 × 3600 seconds
D = (Change in concentration × Distance) / (Time × Average concentration)
D = [(7.4 cm - 7.96 cm) / 7.4 cm] × 1.485 g/cm^3 × 7.4 cm / (10 × 3600 seconds × Average concentration)
To calculate the binary diffusion coefficient, we need to convert the concentration from g/cm³ to moles/m³ by dividing by the molar mass of chloroform.
Molar mass of chloroform (CHCl₃) = 119.37 g/mol
Average concentration in moles/m³ = Average concentration / (Molar mass × 1000 cm³/m³)
Diffusion coefficient = [(7.4 cm - 7.96 cm) / 7.4 cm] × 1.485 g/cm³ × 7.4 cm / (10 × 3600 seconds × (-9.41 x 10^-7 mol/m³))
Finally, substitute the values and calculate the binary diffusion coefficient.
Read more such numericals here: https://brainly.com/question/16646969
#SPJ11
What law did scientists use to predict the Cosmic Microwave Background Radiation before it was discovered?
full paragrafth pls
Answers
Penzias and Wilson had discovered the first observational proof in favor of the Big Bang theory of the universe's genesis. They split the 1978 Nobel Prize in Physics for this discovery.
The radiation temperature of the cosmos has been more precisely calculated to be 2.73 K from further studies of the microwave background at various wavelengths. This is almost half of what Alpher and Herman calculated in 1948, but given the approximations needed for the computation, their conclusion is generally regarded as a successful forecast. The majority of astronomers adopted the Big Bang theory after learning about the cosmic microwave background radiation.
In 1965, the CMB radiation was unintentionally found. American radio astronomers Penzias and Wilson detected a signal in their radio telescope that was impossible to assign to a specific source in the sky. Day or night, summer or winter, it seemed to have the same intensity everywhere.
To learn more about cosmic microwave background radiation refer the link:
https://brainly.com/question/28197648
#SPJ1
calculate the number of nitrate ions present in an 800.0 ml aqueous solution containing 22.5 g of dissolved aluminium nitrate.
Answers
The number of nitrate ions present in an 800.0 ml aqueous solution containing 22.5 g of dissolved aluminium nitrate is 1.91 × 10²³.
To calculate the number of nitrate ions present in an aqueous solution of aluminum nitrate, we first need to determine the number of moles of aluminum nitrate using its molar mass. The molar mass of aluminum nitrate (Al(NO₃)₃) is:
Al: 26.98 g/mol
N: 14.01 g/mol
O: 16.00 g/mol
Molar mass of Al(NO₃)₃ = (26.98 g/mol) + 3 * [(14.01 g/mol) + (16.00 g/mol)] = 26.98 g/mol + 3 * 30.01 g/mol = 213.00 g/mol
Next, we can calculate the number of moles of aluminum nitrate (Al(NO₃)₃) in the solution using its mass:
moles = mass / molar mass
moles = 22.5 g / 213.00 g/mol
moles = 0.1059 mol
Since aluminum nitrate dissociates in water to form one aluminum ion (Al⁺³) and three nitrate ions (NO₃⁻), the number of nitrate ions will be three times the number of moles of aluminum nitrate:
Number of nitrate ions = 3 * moles of Al(NO₃)₃
Number of nitrate ions = 3 * 0.1059 mol
Number of nitrate ions = 0.3177 mol
Finally, to convert the number of moles of nitrate ions to the number of nitrate ions in the solution, we can use Avogadro's number (6.022 × 10²³ ions/mol):
Number of nitrate ions = moles of nitrate ions * Avogadro's number
Number of nitrate ions = 0.3177 mol * 6.022 × 10²³ ions/mol
Number of nitrate ions = 1.91 × 10²³ ions
Therefore, there are approximately 1.91 × 10²³ nitrate ions present in an 800.0 ml aqueous solution containing 22.5 g of dissolved aluminum nitrate.
To know more about aluminium nitrate here
https://brainly.com/question/79967
#SPJ4
o-linked oligosaccharides are commonly attached to the —oh group of
Answers
O-linked oligosaccharides are commonly attached to the —oh group of amino acids.
Glycosylation is a post-translational modification of proteins in which glyco-linked oligosaccharides are added to proteins. This process is found in eukaryotes, primarily in the endoplasmic reticulum and Golgi apparatus, and involves the enzymatic attachment of sugar units to specific sites on a protein molecule.
These short chains of saccharide sugar residues, called glycosyl sidechains, are examples of glycoconjugates which are covalently attached to hydroxyl groups on the protein.
The most common types of glycosylation sites are the N-linked glycosylation sites, which have an attached sugar group, often an N-acetylglucosamine or N-acetylmannosamine connected to an asparagine sidechain, and the O-linked glycosylation sites, which have a sugar group, usually a galactose or mannose sugar, attached to a hydroxyl group on serine, threonine or hydroxylysine residues.
know more about oligosaccharides here
https://brainly.com/question/33448934#
#SPJ11
According to the following reaction, how many moles of iron(III) chloride will be formed upon the complete reaction of 26.2 grams of iron with excess chlorine gas ?
iron(s) + chlorine(g) iron(III) chloride(s)
Answers
Answer:
0.469 mol FeCl₃
Explanation:
Your reaction as a chemical equation is:
2 Fe (s) + 3 Cl₂ (g) ⇒ 2 FeCl₃ (s)
You have 26.2 grams of iron. Convert to moles of iron.
(26.2 g)/(55.845 g/mol) = 0.4692 mol
Use the mole ratio from the chemical equation to convert moles of iron to moles of iron(III) chloride.
(0.4692 mol Fe) × (2 mol FeCl₃/2 mol Fe) = 0.469 mol FeCl₃
You will have 0.469 moles of iron(III) chloride at the end of the reaction.
0.469 moles of iron(III) chloride upon the complete reaction of 26.2 grams of iron with excess chlorine gas
What do you mean by Moles?Its a typical scientific unit used to measure vast numbers of extremely small objects, such as atoms, molecules, or other specific particles.
Iron content in you is 26.2 gram. Transform into moles of iron.
(0.4692 mole) = (26.2 g)/(55.845 g/mole)
Converting moles of iron to moles of iron(III) chloride requires using the mole ratio from the chemical equation.
(2 mole FeCl3/2 mole Fe) (0.4692 mole Fe) = 0.469 mole FeCl3
.
After the process, you will possess 0.469 moles of iron(III) chloride.
To learn more about Moles, refer to the below link:
https://brainly.com/question/15209553
# SPJ2
In the summer, young animals grow bigger to give them a chance of surviving the winter. How does this compare to how a plant responds to the change in season?
Young plants grow seeds.
Young plants go dormant.
Young plants grow bigger.
Young plants drop their leaves.
Answers
Answer:
B
Explanation:
They go into a state of dormancy like hibernation .
In this stage they do
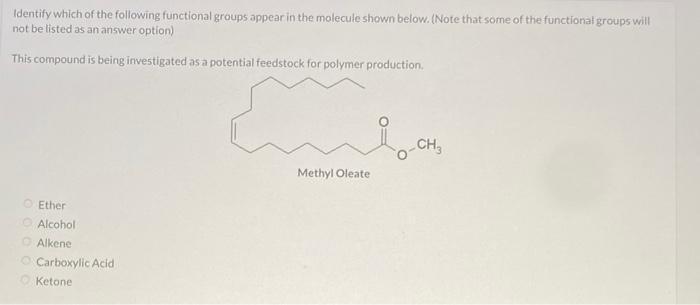
Metabolism slow heart rate lower body tempratureidentify which of the following functional groups appear in the molecule shown below. (note that some of the functional groups will not be listed as an answer option)
Answers
The functional groups that we have in the compound are ketone and alkene. Options C and E
What is a functional group?
A functional group is a particular set of atoms in a molecule that are in charge of the molecule's distinctive chemical processes and characteristics. The behavior and functionality of a molecule in chemical processes are determined by a reactive component of the molecule.
The common atoms found in functional groups are carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorus. They can be recognized by their unique atom arrangement and bonding structure inside a molecule.
Learn more about functional group:https://brainly.com/question/1356508
#SPJ4
A 30.0 L container was filled by pumping into it 10.0 L of CO2 gas at 60.0 torr of pressure and 20.0 L of O2 gas at 50.0 torr of pressure. All volumes and pressures were measured at the same temperature. What is the final pressure of CO2 in this mixture?
Answers
The final volume of the Oxygen O2 would simply be equivalent to the overall volume of the final mixture. This is because gases would occupy every space of its container. So in this case, the volume of CO2 and O2 would be the same and it would be 30 L. What would be different is the partial pressures of each.
Answer:
30 L
determine the total hardness as caco3 for a sample of water with a calcium content of 50 milligrams per liter (mg/l) and a magnesium content of 10 mg/l.
Answers
The total hardness as measured by caco3 for a sample of water with a 50 mg/l calcium content and a 10 mg/l magnesium content is 166.67 mg/L.
Total hardness is a measurement of a water sample's mineral content that cannot be removed by boiling. More specifically, the quantity of multivalent cations in the water determines total hardness. These cations are positively charged in excess of 1+. Cations typically have a charge of 2+. Mg2+ and Ca+ are the two most frequent cations to be found in hard water.
\(Total hardness= \frac{[Ca^{++}]mg/L }{Equivalent weight of Ca} * Equivalent weight of CaCo_3 + \frac{[Mg^{++} ]mg/L}{Equivalent weight of Mg} * Equivalent weight of CaCo_3\)
\(Total hardness= \frac{50}{20} *50+ \frac{10}{12} *50\\Total hardness= \frac{125}{1}+ \frac{125}{3}\)
Taking LCM of 1 and 3,
\(Total hardness= \frac{375+125}{3} \\Total hardness= \frac{500}{3} \\Total hardness=166.67\)
To learn more about water, refer:-
https://brainly.com/question/28465561
#SPJ4
1s22s22p63s23p3 atoms of an element, x, have this electron configuration. The compound most likely formed with magnesium, Mg, is
A. MgX
B. Mg2X
C. MgX2
D. Mgx3
E. MgX2
Answers
Answer:
Mg3X2 is correct answerrrrrr
Ca2+ ions (essential for contraction) are stored in the
a. sarcoplasm
b. sarcolemma
c. sarcoplasmic reticulum
d. T-tubules
Answers
The correct answer is c. sarcoplasmic reticulum. Ca2+ ions, which are essential for muscle contraction, are stored in the sarcoplasmic reticulum (SR) of muscle cells.
The sarcoplasmic reticulum is a specialized network of membranous sacs within muscle fibers, specifically designed for the storage and release of calcium ions during muscle contraction.
When a muscle is stimulated, an action potential triggers the release of stored Ca2+ ions from the sarcoplasmic reticulum into the sarcoplasm, the cytoplasm of muscle cells. The influx of Ca2+ ions into the sarcoplasm initiates a series of events leading to muscle contraction.
The sarcoplasm refers to the cytoplasm of muscle cells, the sarcolemma is the plasma membrane of muscle cells, and T-tubules are invaginations of the sarcolemma that help transmit the action potential deep into the muscle fiber.
Therefore, the correct location where Ca2+ ions are stored for muscle contraction is the sarcoplasmic reticulum (c).
To learn more about contraction
https://brainly.com/question/1166774
#SPJ11
what is the mass of 0.083 moles of iron to the nearest tenth
Answers
Answer:
The answer is 0.01790670606142. We assume you are converting between moles Iron and gram.
Explanation:
The mass of 0.083 moles of iron to the nearest tenth is 0.4635135 grams.
What is mass?Mass is defined as the amount of substance contained in a particle or object as a dimensionless quantity. An object's mass is the entire amount of matter it contains. A scalar quantity, it. By using beam balance, it is measured. The object's mass is constant everywhere it is. The inertia of an object allows us to calculate its mass.
One carbon-12 atom weighs exactly 12 atomic mass units, which equals its atomic mass. One mole of carbon-12 atoms weighs exactly 12 grams; this is known as the molecular mass of carbon-12.
Molar mass of iron is 55.847 moles
Molar mass = mass/mole
Mass = molar mass x mole
Mass = 55.845 x 0.083
Mass = 0.4635135 grams.
Thus, the mass of 0.083 moles of iron to the nearest tenth is 0.4635135 grams.
To learn more about mass, refer to the link below:
https://brainly.com/question/19694949
#SPJ2
A scientist discovers a deep bowl-like divot under the ocean off the coast of eastern Mexico that is many kilometers across. The layers of the ground all around the continent from around the time that structure formed contain large amounts of iridium and a larger number of fossils than is normally found. What most likely caused the bowl-like structure?
a meteorite impact
an asteroid impact
an iridium spike
a Kuiper object impact
Answers
Answer:
an asteroid impact or B
Explanation:
An asteroid impact most likely caused the bowl-like structure. Hence, option B is correct.
What is divot or bowl like structure?A divot are the marks made in the grass on the golf course. So, scientist named a bowl like structure form in under the ocean off the coast of eastern Mexico.
An impact event is a collision between astronomical objects causing measure effect.If the asteroid hits on land of the earth there will be the huge amount of dust thrown up into the atmosphere.If it hits water, then there would be an increase in water in the atmosphere.Example: the impact of asteroid ended the age of dinosaurs a 66 million years ago.
It kills more than 75 percent of earth species.The asteroid impact in ocean made bowl type structure called divot these kilometers in size so big as compared to a football ground.
An asteroid impact most likely caused the bowl-like structure. Hence, option B is correct.
Learn more about asteroid impact. here:
https://brainly.com/question/24233378
#SPJ6
A ample of ga at 20ºC ha a volume of 10 L and exert a preure of 912 mm Hg. How many mole
of ga are in the ample?
a. 0. 3 mol c. 0. 8 mol
b. 0. 5 mol d. 1. 00 mol
Answers
The Number of moles present is 0.5mol.
What is ideal gas law?
The general gas equation, also known as the ideal gas law, is the equation of state for a fictitious ideal gas. The equation for the ideal gas's relationship to pressure P, volume V, temperature T, and number of moles n is known as the ideal gas law.
Use P V = n R T
R = 0.08206 L -atm /mol -K when the pressure is in atmosphere atm, the volume is in litres L, and the temperature is in Kelvin K.
One atm is equal to 760.0 mm Hg, so depending on how the pressure changes, there will either be a division or multiplication.
Divide the value in mmHg by 760.0 mmHg/atm to convert it to atm.
P= 912mmHg /760mmHg/atm=1.2atm
to convert °C to kelvin,
K=°C +273= 20+273=293K
R=0.08206
Add these values to given equation,
n=PV/RT
= 1.2×10÷0.08206×293
= 12÷24.02
=0.5mol
To learn more about ideal gas law
https://brainly.com/question/27870704
#SPJ4
How many moles of Al are necessary to form 66.8 g of AlBr₃ from this reaction:
2 Al(s) + 3 Br₂(l) → 2 AlBr₃(s) ?
Answers
0.1250 moles of Al are necessary to form 66.8 g of AlBr₃.
To solve this problem, we need to use the molar mass of AlBr₃ to convert the given mass to moles, and then use the stoichiometry of the balanced chemical equation to determine the moles of Al required.
First, we calculate the molar mass of AlBr₃ by adding the atomic masses of its constituent elements:
Al = 1 × 26.98 g/mol = 26.98 g/mol
Br = 3 × 79.90 g/mol = 239.70 g/mol
Total molar mass = 26.98 + 239.70 = 266.68 g/mol
Next, we can use the given mass of AlBr₃ and its molar mass to determine the number of moles:
moles of AlBr₃ = mass / molar mass
moles of AlBr₃ = 66.8 g / 266.68 g/mol
moles of AlBr₃ = 0.2500 mol
Finally, we can use the stoichiometry of the balanced chemical equation to relate the moles of AlBr₃ to the moles of Al:
2 Al(s) + 3 Br₂(l) → 2 AlBr₃(s)
2 mol Al = 3 mol Br₂ = 2 mol AlBr₃
moles of Al = (moles of AlBr₃ / 2) × (2 mol Al / 2 mol AlBr₃)
moles of Al = 0.2500 mol / 2
moles of Al = 0.1250 mol
Therefore, 0.1250 moles of Al are necessary to form 66.8 g of AlBr₃.
Learn more about molar mass here:
https://brainly.com/question/22997914
#SPJ11
The theoretical yield and the percent yield are calculated shown below. Did you perform the calculations correctly?

Answers
Answer:
\(56 \times { \frac{01514344}{?} }^{2} 5566648443hffii51 \\ \div 232333\)
Answer:
write a letter to the presiding member of your district assessment telling him or her about two of the achievement of your community over the last five years and the plans for the future
Uranium contains two isotopes, U-235 with an atomic mas of 235 g/mol, and u-238 with an atomic mass of 238g/mol. U-235 is needed
as a fuel in nuclear reactors. Until recently, the method used to separate U-235 from U-238 was by gas diffusion. Use U-235 as R1, and
U-238 as R2 and determine the rate of diffusion and which gas will diffuse faster.
⚪︎U-235 diffused 10.01 times faster than U-238
⚪︎U-235 diffused 1.01 times slower than U-238
⚪︎U-235 effused 2.01 times slower than U-238
⚪︎U-235 effused 1.01 times faster than U-238
⚪︎U-235 diffused 1.01 times faster than U-238

Answers
Answer:
the answer is in the image
Explanation:

why does the hydrogen gas need to flow continuously for a while before starting the heating process?
Answers
In the laboratory, hydrogen gas is used as fuel for various purposes, including heating. In order to start the heating process, it is necessary to allow the hydrogen gas to flow continuously for a while. This is because there may be air or other gases present in the hydrogen gas pipeline that can affect the heating process.
When the hydrogen gas is allowed to flow continuously for a while, the air or other gases are purged from the pipeline, which improves the quality of the hydrogen gas. This ensures that there is no interference with the heating process, which could otherwise lead to inaccurate results.The continuous flow of hydrogen gas is essential because if it is not allowed to flow for a while, air or other gases can cause damage to the burner or other equipment used for heating. The air or other gases can cause an explosion, which can result in severe injury or death.In conclusion, the hydrogen gas needs to flow continuously for a while before starting the heating process to remove any air or other gases from the pipeline. This improves the quality of the hydrogen gas, ensures accurate results, and prevents damage to the equipment. It is important to follow safety protocols when using hydrogen gas to prevent any accidents.For such more question on heating process
https://brainly.com/question/29317333
#SPJ8
What form of chemical weathering is responsible for breaking the serpentinite down on Ruby Jones Hall
Answers
The form of chemical weathering that is responsible for breaking the serpentinite down on Ruby Jones Hall is hydrolysis.
Hydrolysis is a type of chemical weathering that occurs when minerals in rocks react with water and create new compounds as a result. It is particularly important in the weathering of silicate minerals, including the serpentinite found on Ruby Jones Hall. During hydrolysis, water molecules split into hydrogen and hydroxide ions and then react with the minerals. This reaction alters the minerals and creates new ones, often resulting in a softer, weaker rock that is more easily eroded. The process of hydrolysis breaks down the serpentinite on Ruby Jones Hall. Serpentinite is a rock made primarily of the mineral serpentine.
Serpentine is a magnesium-rich mineral that is susceptible to hydrolysis because it reacts readily with water to form other minerals. When water reacts with serpentine, it breaks down the mineral and produces new minerals, including clay minerals like kaolinite and smectite. These new minerals are much softer and more easily eroded than the original serpentine, which is why serpentinite is often found in areas with high rates of weathering and erosion.
To know more about hydrolysis refer to:
https://brainly.com/question/30718479
#SPJ11