A chemist reacted 0. 2 moles sodium benzoate with 0. 25 moles of hydrochloric acid. if she generated 22 g benzoic acid, what was her percent yield? (mw of benzoic acid = 122. 12 g mol-1)
Answers
A chemist reacted 0. 2 moles sodium benzoate with 0. 25 moles of hydrochloric acid. if she generated 22 g benzoic acid, the percent yield is 90%.
Reactant + HCl ------ Product
0.2 mol 0.25 22g
mol
Number of moles of reactant = 0.2mol
Molar mass of product = 122.12g
Number of moles of product = given mass/ molar mass
= 22g/ 122.12 g
= 0.180mol
Percent yield = (number of moles of product/ number of moles of reactant) × 100
= (0.180/0.2) × 100
= 90%
Thus we concluded that the percent yield of the given solution is 90%.
learn more about percent yield:
https://brainly.com/question/17042787
#SPJ4
Related Questions
What is the freezing point of a solution that contains 10.0 g of glucose in 100g of H2O?
Answers
The proposed solution, which has 10.0 grams of glucose in 100 gram of water, has a freezing point of 1.034 C.
The freezing point is what?The degree of heat in which a liquid becomes solid. precisely the temperatures at which a material's solid and liquid states are balanced at atmospheric pressure.
How significant is freezing point?If a substance is kept below its freezing point, it may either become more or less dangerous. The freezing point additionally offers an essential safety standard for evaluating the impacts of occupational exposure to cold conditions.
Briefing:m = molality
i = van 'toff factor,
molality = n/w*t of solvent
n = w*t of Glucose/M* w t
= 10/180*1/0.1
=0.555 w* t
= 1.86*0.555*1
= 1.034
T (solvent) - d= 0-1.034
freezing point = -1.034C
To know more about freezing point visit:
https://brainly.com/question/9530198
#SPJ4
Which of these peptides is positively charged, which is
negatively charged, and which is neutral at physiological pH? What
is the charge on each peptide?
SDEKAINVKWQLA
SDEKAINVKWQHA
SEERAINVAWQHA
SDEK
Answers
This peptide is positively charged at physiological pH. In conclusion, SDEKAINVKWQLA and SDEKAINVKWQHA are neutral at physiological pH, SEERAINVAWQHA is negatively charged, and SDEK is positively charged.A peptide is a short chain of amino acids that are joined together with peptide bonds.
The nature of a peptide's charge depends on the overall charges of the amino acids in the sequence. At physiological pH, amino acids will either be positively charged, negatively charged, or neutral, depending on their side chains. Let's examine the peptides provided to determine their charges at physiological pH:SDEKAINVKWQLA: This peptide contains a mix of amino acids with positively charged, negatively charged, and neutral side chains. However, the positively charged amino acid (lysine) and the negatively charged amino acid (aspartic acid) are present in equal amounts.
Therefore, this peptide is considered neutral at physiological pH.SDEKAINVKWQHA: This peptide is similar to the first one but has one less amino acid (alanine instead of leucine at the end). It contains the same number of positively charged and negatively charged amino acids, and so it is also neutral at physiological pH.SEERAINVAWQHA: This peptide contains three negatively charged amino acids (aspartic acid and glutamic acid) and only one positively charged amino acid (lysine). Therefore, the peptide overall is negatively charged at physiological pH.SDEK: This peptide contains both positively charged (lysine) and negatively charged (aspartic acid) amino acids. However, there are more positively charged amino acids in the peptide than negatively charged ones.
To know more about peptide visit:-
https://brainly.com/question/32004016
#SPJ11
How many kilojoules of energy must be added to 69.0 grams of water to change the temperature from 33.0°C to 95.0°C? (CH2O = 4.184 j/g oc, 1 kj = 1000 j)
Answers
To calculate the amount of energy required to change the temperature of water, we can use the formula: Q = m * c * ΔT Where: Q is the amount of energy in joules, m is the mass of the water in grams.
c is the specific heat capacity of water in joules per gram per degree Celsius
ΔT is the change in temperature in degrees Celsius
Given:
m = 69.0 grams
c = 4.184 j/g°C
ΔT = (95.0 - 33.0)°C
= 62.0°C
First, let's convert the mass of water to kilograms by dividing it by 1000:
m = 69.0 grams ÷ 1000
= 0.069 kg
Now, substitute the values into the formula:
Q = 0.069 kg * 4.184 j/g°C * 62.0°C
Simplifying the equation:
Q = 0.069 kg * 4.184 j/g°C * 62.0°C
Q = 0.069 kg * 4.184 j/g°C * 62.0°C
Q = 0.069 kg * 260.368 j/g
To convert the result from joules to kilojoules, divide by 1000:
Q = (0.069 kg * 260.368 j/g) ÷ 1000
Q = 18.0 kj
Therefore, 18.0 kilojoules of energy must be added to 69.0 grams of water to change the temperature from 33.0°C to 95.0°C.
To calculate the amount of energy required to change the temperature of water, we use the formula Q = m * c * ΔT. In this formula, Q represents the amount of energy in joules, m is the mass of the water in grams, c is the specific heat capacity of water in joules per gram per degree Celsius, and ΔT is the change in temperature in degrees Celsius.
Given that m = 69.0 grams,
c = 4.184 j/g°C,
and ΔT = (95.0 - 33.0)°C
= 62.0°C, we can substitute these values into the formula.
First, we convert the mass of water to kilograms by dividing it by 1000. Then, we multiply the mass, specific heat capacity, and change in temperature to calculate the energy in joules. Finally, we convert the result from joules to kilojoules by dividing by 1000. The final answer is that 18.0 kilojoules of energy must be added to 69.0 grams of water to change the temperature from 33.0°C to 95.0°C.
The energy required to change the temperature of 69.0 grams of water from 33.0°C to 95.0°C is 18.0 kilojoules.
To know more about temperature visit:
brainly.com/question/7510619
#SPJ11
Approximately 0.183 kilojoules of energy must be added to the water to change the temperature from 33.0°C to 95.0°C.
To calculate the amount of energy needed to change the temperature of the water, we can use the formula:
q = m * c * ΔT
Where:
q is the energy required (in kilojoules)
m is the mass of the water (in grams)
c is the specific heat capacity of water (4.184 J/g°C)
ΔT is the change in temperature (in °C)
First, we need to convert the mass of water from grams to kilograms by dividing by 1000:
69.0 grams ÷ 1000 = 0.069 kg
Next, we can calculate the change in temperature:
ΔT = final temperature - initial temperature
ΔT = 95.0°C - 33.0°C = 62.0°C
Now, we can substitute the values into the formula:
q = 0.069 kg * 4.184 J/g°C * 62.0°C
To convert the answer to kilojoules, we divide by 1000:
q = (0.069 kg * 4.184 J/g°C * 62.0°C) ÷ 1000
Simplifying the equation:
q = 0.1826 kJ
Therefore, approximately 0.183 kilojoules of energy must be added to the water to change the temperature from 33.0°C to 95.0°C.
Learn more about Solution here:
https://brainly.com/question/33921240
#SPJ11
Potassium and fluorine are both halogens?
Answers
Answer:
false, Potassium and fluorine are not halogens.
only fluorine here is halogen.
potassium is an alkali earth metal it doesn't comes under category of halogens, but fluorine
is a non metal which comes under halogen family.
How is latitude related to temperature?
Answers
Answer:
The closer a place is to the equator the warmer it is
Explanation:
The equator is the part of the earth that is in the sun the longest and the poles are in the sun for a very short period of time each year. Therefore the equator is the warmest compared to the two poles.
By law, a gallon of ice cream, sold in stores in the US, must have a
weight of at least 4. 5 pounds. Cheap ice cream has a weight of 4. 5
pounds. More expensive ice creams have a mass of 9. 0 pounds. If a
kilogram is about 2. 2 pounds and a gallon is about 3785 milliliters,
what are the densities of the cheap and expensive ice creams?
Answers
The volume of the expensive ice cream is: 0.
Densities of the cheap and expensive ice creams, we need to first convert the weights of the ice creams from pounds to kilograms.
1 pound = 0.453592 kilograms
Therefore, the weight of the cheap ice cream in kilograms is:
5 pounds * 0.453592 kilograms/pound = 2. 027 kilograms
The weight of the expensive ice cream in kilograms is:
0 pounds * 0.453592 kilograms/pound = 3. 903 kilogram
The volume of a gallon of ice cream is approximately 3785 milliliters. Therefore, the volume of the cheap ice cream is:
027 kilograms / 3785 milliliters = 0.000557 cubic meters
The volume of the expensive ice cream is:
903 kilograms / 3785 milliliters = 0.00091 cubic meters
The densities of the cheap and expensive ice creams, we can use the following formula:
density = mass / volume
The densities of the cheap and expensive ice creams can then be calculated using the following formula:
density = mass / volume
The mass of the cheap ice cream is:
027 kilograms
The volume of the cheap ice cream is:
0.000557 cubic meters
Therefore, the density of the cheap ice cream is:
027 kilograms / 0.000557 cubic meters = 35. 14 kilograms/cubic meter
The mass of the expensive ice cream is:
903 kilograms
The volume of the expensive ice cream is: 0.
Learn more about volume Visit: brainly.com/question/27710307
#SPJ4
Two ions have a different number of
Answers
Answer:
Explanation:
Isotopes differ in the number of neutrons; in ions the number of electrons is different from the number of protons. Isotopes are atoms that have the same number of protons but different numbers of neutrons.
Predict the ordering, from shortest to longest, of the bond lengths in CO , CO 2 , and CO3^2-. Rank from shortest to longest. To rank items as equivalent, overlap them.
Answers
The bond lengths are in the following order, shortest to longest: COCO2CO32-
What is bond length ?
The equilibrium separation between two bound atoms' nuclei in a molecule is known as the bond length. Bond multiplicity results in a reduction in bond length. It can be measured using rotational, X-ray, and other spectroscopic techniques.
The triple bond is the shortest and strongest, the double bond is the shortest and weakest, and the single bond is the longest and weakest. As a result, the triple bond in CO makes it the shortest bond, the double bond in CO2 makes it shorter and stronger, and the combination of double and single bonds in CO32 makes it the longest and weakest bond. Carbon-carbon bonds, which are found in diamonds, are thought to be the longest bond length. It lasts for 154 minutes. It is the longest because of the three-dimensional structure of diamond and the covalent bonds that hold the carbon atoms together.
To learn more about bond length click here:
https://brainly.com/question/13067561
#SPJ4
What is the difference between endothermic and exothermic changes?
Which type of change (exothermic or endothermic) is water freezing? Why? What type of matter is made of really hot and super excited (fast-moving) charged particles?
Answers
Answer:
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings. The frezing of water is an exothermic process because heat is being removed from the system. In gases the particles move rapidly in all directions, frequently colliding with each other and the side of the container. With an increase in temperature, the particles gain kinetic energy and move faster. Hope this helped! :)
What is the texture of hydrogen?
Answers
Answer: The texture is one of the most important physical characteristics of the solid or liquid materials.
Texture is actually the physical feel of the surface layer of a material when we physically touch it.
So,by it's definition we came to know that the texture is only possible for the substances which have physical surfaces.
And the gaseous materials don't have any physical surface to feel it's texture.
That's why gaseous materials including hydrogen gas don't have any kind of texture.
Explanation:
Which organelle is responsible for controlling the passage of water, nutrients and waste products into and/or out of the cell in order to help maintain homeostasis?.
Answers
The cell membrane is responsible for controlling the passage of water, nutrients and waste products into and out of the cell in order to maintain homeostasis.
The cell membrane is what?The plasma membrane is also known as cell membrane. The cell membrane, as opposed to the cell wall, is a component of every living thing. The lipid bilayer that makes up the cell membrane is naturally selectively permeable.
Only the chosen or necessary substances can pass across the cell membrane because the material is selectively permeable. As a result, the cell membrane has the ability to control how much water, minerals, and other substances are transported into and out of the cell.
The cell membrane is made up of a variety of proteins in addition to lipids. Either they are transportation-assisting proteins or structural proteins.
A significant amount of cholesterol is also present in the cell's lipids. In addition to protecting the cell by limiting the admission of harmful substances and the expulsion of necessary substances, the cell membrane also gives it structural support.
To know more about Cell membrane, visit:https://brainly.com/question/18663048
#SPJ4
what element is being oxidized in the following redox reaction
Answers
Without the specific redox reaction, we cannot determine the element being oxidized.
In the given redox reaction, we need to determine which element is being oxidized. To do this, we compare the oxidation states of the elements before and after the reaction.
Unfortunately, the specific redox reaction is not provided in the question. Without the reaction, we cannot determine the element being oxidized. Please provide the specific redox reaction so that we can analyze it and identify the element being oxidized.
Learn more:About element here:
https://brainly.com/question/14347616
#SPJ11
what is the density of a gas at STP that has a molar mass of 50.0 g/mol?
Answers
Answer:
2.232 g/L
Explanation:
Assuming 1 mol, volume at STP is 22.4 L so you simply divide 50g by 22.4 L to get density
The density of the given gas is required.
The density of the gas at STP is 2.232 g/L.
M = Molar mass of gas = 50 g/mol
1 mole of any gas at STP occupies 22.4 L.
V = Volume per mole = 22.4 L/mol
Density is given by
\(\rho=\dfrac{M}{V}\\\Rightarrow \rho=\dfrac{50}{22.4}\\\Rightarrow \rho=2.232\ \text{g/L}\)
Learn more:
https://brainly.com/question/8684338
https://brainly.com/question/12247942
Which molecules show an increase in bond order when one electron is added to the molecule?.
Answers
\(C_{2}\) and \(B_{2}\) molecules show an increase in bond order when one electron is added to the molecule.
When an electron is added to \(C_{2}\) molecule, the bond order increases from 2 to 2.5 as the electron is added into σ\((2Px)\) orbital which is bonding in nature. This leads to stabilization.
\(B_{2}\) has 2 unpaired electrons because of the single occupancy of the degenerate pi orbitals with a bond order one.
Adding one electron to give monoanionic results in pairing one of these electrons leaving one unpaired electron.
The bond order increases to 1.5.
To learn more about bond order visit: https://brainly.com/question/29057354
#SPJ1
III. Problem solving If a 20 W/0.020 kWh table lamp is used for 10 hours, how much electrical energy is consumed? Given:
Find:
Required: energy used
Equation:
Solution:
Answer:
Answers
By multiplying the power of the table lamp (20 W) by the time of use (10 hours), we find that the lamp consumes 200 watt-hours (Wh) of energy. Converting this value to kilowatt-hours (kWh), we get the final answer of 0.2 kWh.
To find the electrical energy consumed by the table lamp, we can use the equation:
Energy = Power x Time
Given:
Power of the table lamp = 20 W
Time of use = 10 hours
Substituting these values into the equation, we get:
Energy = 20 W x 10 hours
Now, let's calculate the energy:
Energy = 200 watt-hours (Wh)
However, the answer is given in terms of kilowatt-hours (kWh). To convert from watt-hours to kilowatt-hours, we divide the value by 1000:
Energy = 200 Wh / 1000 = 0.2 kWh
Therefore, the amount of electrical energy consumed by the table lamp is 0.2 kilowatt-hours (kWh).
Know more about electrical energy here:
https://brainly.com/question/874116
#SPJ8
which metals would act as sacrificial anodes (cathodic protection) for iron? na cr mg sn cu despite its ability to act as a sacrificial anode for iron, which metal's reactivity with water makes it unsuitable to attach to the hull of a steel ship? cr na sn mg cu
Answers
Magnesium and Zinc would act as sacrificial anodes for iron in cathodic protection.
Sacrificial anodes are more reactive metals that are attached to the metal that needs protection (in this case, iron) to prevent corrosion. As the more reactive metal corrodes instead, it protects the iron from corroding. Magnesium and Zinc are both more reactive than iron, which makes them suitable as sacrificial anodes for iron in cathodic protection.
Despite its ability to act as a sacrificial anode for iron, Copper's reactivity with water makes it unsuitable to attach to the hull of a steel ship. Copper reacts with water to form copper oxide, which can create a layer on the surface of the copper and prevent further reaction. This means that the copper would not corrode as quickly as it needs to in order to effectively protect the iron. Therefore, it is not a good choice as a sacrificial anode for iron in cathodic protection on a steel ship's hull. Chromium, Sodium, and Tin are also not suitable as sacrificial anodes for iron due to their lower reactivity than iron.
To know more about cathodic protection visit:
https://brainly.com/question/4249019
#SPJ11
Ainsley wanted to know if caffeine impacted the rate a mouse learned a maze. He tested two groups of mice and timed them as they attempted to learn the maze. Lab group A received 5 mL of caffeine with their 20 g of grain. Lab group B received 20 g of grain without caffeine. Which is Ainsley's independent variable?
Answers
Answer:
Caffeine
Explanation:
In every study, there must be an independent variable and a dependent variable. The independent variable is being manipulated and its effect on the dependent variable is evaluated.
In this study, the independent variable is the administration of caffeine. Its effect on a rat's ability to learn the maze (dependent variable) is evaluated in the study.
Draw the product of the complete hydrogenation of ethyne. Draw the molecule on the canvas by choosing buttons from the Too default. Include all hydrogen atoms.
Answers
The product of the complete hydrogenation of ethyne is ethane (C2H6), where all carbon-carbon triple bonds (C≡C) are converted to carbon-carbon single bonds (C-C), and each carbon atom is bonded to three hydrogen atoms.
To draw the product of the complete hydrogenation of ethyne, we start with the molecular formula of ethyne, which is C2H2. Ethyne consists of two carbon atoms and two hydrogen atoms, with a triple bond between the carbon atoms.
To complete the hydrogenation, we need to add hydrogen atoms to the carbon atoms to convert the triple bond to a single bond. Since each carbon atom in ethyne needs two additional bonds to reach a stable configuration, we add two hydrogen atoms to each carbon atom.
After adding the hydrogen atoms, we have two carbon atoms, each bonded to three hydrogen atoms, resulting in the formation of ethane (C2H6). In ethane, all carbon-carbon triple bonds are converted to carbon-carbon single bonds, and each carbon atom is bonded to three hydrogen atoms.
Therefore, the final product of the complete hydrogenation of ethyne is ethane (C2H6), where all hydrogen atoms are included in the molecule.
To know more about the hydrogenation of ethyne click here:
https://brainly.com/question/14697157
#SPJ11
Calculate the number of copper atoms in 2.45 mole of copper
Answers
Answer:
1.48×10²⁴ Cu atoms
Explanation:
For this question you need to use Avogadro's number 6.022×10²³atoms.
2.45 moles of Cu ×\(\frac{6.022x10x^{23} atoms }{1 mole}\)= 1.47539×10²⁴ atoms.
The moles cancel out so you are left with atoms.
Since there are 3 significant figures in the question there should be 3 significant figures in your answer, which is 1.48×10²⁴ Cu atoms.
In general, foods high in saturated fatty acids are found in which food groups?
Select one:
a. Fruits and vegetables
b. Milk and meat
c. Vegetable oils
d. Starches and breads
Answers
In general, foods high in saturated fatty acids are found in milk and meat food groups (Option B).
What are saturated fats?Saturated fats are those that are solid at room temperature and have no double bonds between their carbon atoms. Saturated fats are commonly found in foods of animal origin, such as fatty beef, pork, and poultry, as well as in butter, cheese, and other dairy products.
Lipids that are solid at ambient temperature are known as saturated fatty acids. They are present in meat and dairy products, including cheese, milk, and butter, as well as some plants, including coconut oil, cocoa butter, and palm oil. Foods high in saturated fats are usually of animal origin and are linked to an increased risk of heart disease.
Thus, the correct option is B.
Learn more about Saturated fats: https://brainly.com/question/29816448
#SPJ11
1. Explain the differences between the "lonic Bond" and "Covalent Bond". 2. 500 grams of sugar occupies a volume of 0.315 L. What is the density of the sugar in g/cm^3 ? 3. What is the mass of a 450 m^3 block of silicon if the density of silicon is 2.336 g/cm^3 ?
Answers
1. The ionic bond and covalent bond are distinguished from each other based on the way the atoms are attached to each other. Ionic bonds are formed when one or more electrons are transferred from one atom to another, resulting in a cation and an anion attracting each other to form an ionic bond. Ionic bonds are a result of electrostatic attraction between ions with opposite charges. Covalent bonds are formed when atoms share one or more pairs of electrons to achieve stable electronic configurations. Covalent bonds form when two or more atoms share electrons in order to achieve the stable electron configuration of noble gases. In covalent bonding, the electrons are shared between atoms, not transferred.
2. Density is defined as mass per unit volume. The density of sugar in g/cm³ is obtained by dividing its mass by its volume.
Mass of sugar = 500gVolume of sugar = 0.315L
Using the formula for density, we have
Density = Mass/Volume= 500g/0.315
L= 1587.30 g/L1
L = 1000 mL1 mL = 1 cm³
Density = 1587.30 g/L × (1 mL/1 cm³)
Density = 1.5873 g/cm³, to 4 significant figures
3. We can use the formula; Density = Mass/Volume, to calculate the mass of the block of silicon. Volume of block of silicon = 450 m³ Density of silicon = 2.336 g/cm³ Volume of block of silicon = 450 m³ = 4.5 × 10^8 cm³
We can substitute the values into the formula
Density = Mass/Volume
2.336 g/cm³ = Mass/4.5 × 10^8 cm³
Mass = 2.336 g/cm³ × 4.5 × 10^8 cm³
Mass = 1051200000 g or 1.05 × 10^9 g, to 2 significant figures.
Therefore, the mass of the block of silicon is 1.05 × 10^9 g.
To know more about density visit:
https://brainly.com/question/29775886
#SPJ11
How many milliliters of a 0.80mM ammonium hydroxide solution are required to create 2.0L of 0.30mM solution? Do not include units in the answer. Your answer should have two significant figures.
Answers
To make 2.0L of a 0.30mM solution, \(6.4 \times 10^3\) millilitres of a 0.80mM ammonium hydroxide solution are needed.
What is ammonium hydroxide?An aqueous solution containing ammonium (\(NH_4\)) and hydroxide (\(OH^-\)) ions is known as ammonium hydroxide. Because it is a strong base, it totally dissociates in aqueous solutions, resulting in an alkaline reaction.
Ammonium hydroxide is utilised in a wide range of commercial and domestic applications, including the production of cleaning agents. It can be used for cleaning, deodorising, and bleaching, among other things. It serves as a dough conditioner and an
acidity regulator in the food sector.
\(6.4 \times 10^3\)
Let x = 0.80 mM ammonium hydroxide solution in millilitres is needed.
We know that molarity (mM) = moles/L
Given:
\(M_1\) = 0.80 mM
\(M_2\) = 0.30 mM
\(V_2\) = 2.0 L
As a result, we may construct the equation shown below:
(0.80 mM)(x mL) / (1000 mL/L) = (0.30 mM)(2.0 L)
Solving for x, we get:
x = \(6.4 \times 10^3\) mL
In order to produce 2.0L of 0.30mM solution, \(6.4 \times 10^3\) mL of a 0.80mM ammonium hydroxide solution are needed.
To learn more about ammonium hydroxide, visit:
brainly.com/question/31367127
#SPJ1
write the chemical equation that describes the reaction of room temperature titanium tetrachloride with water vapor to produce a titanium dioxide smokescreen. include phases.
Answers
To shed light on the process by which titanium dioxide (TiO2) nanoparticles are formed, titanium tetrachloride (TiCl4) hydrolysis has been investigated.
What takes place when TiCl4 is dissolved in water?Hydrogen chloride (HCl) gas, which is extremely caustic and hazardous, is known to be released violently when TiCl4 reacts with water. The estimate of the scope and gravity of the effects of large accidents that have been identified, such as an unintentional spill of TiCl4, is, however, not shared by the industry.
Why is room temperature titanium chloride liquid?An alternative strategy (which is the opposite of the solution above) would be to assert that since metal chlorides are often ionic, titanium chloride must be a tiny (covalent) molecule with weak molecular interactions if it is a liquid at room temperature.
to know more about titanium dioxide here:
brainly.com/question/16978406
#SPJ4
What is the maximum possible amount of product obtained in a chemical reaction?.
Answers
Answer:
theoretical yield
Explanation:
Theoretical yield is the maximum possible amount of product obtained in a chemical reaction .
What do you mean by the theoretical yield ?Theoretical yield is the quantity of a product obtained from the complete transformation of the limiting reactant in a chemical reaction.
Theoretical yield is commonly expressed in terms of grams or moles.
It can be calculated from-:
The balanced chemical equation, the mass and relative formula mass of the limiting reactant, and the relative formula mass of the product.
Hence ,theoretical yield is the maximum possible amount of product obtained in a chemical reaction .
Learn more about theoretical yield ,here:
https://brainly.com/question/20522162
#SPJ6
(a) Write down the main inorganic carbon system reactions in seawater, starting from the transfer of CO2 from the air phase into the water phase, and the associated chemical equilibrium equations. Briefly explain what each term in the equations is. [3 Points]
(b) Manipulate the chemical equilibrium equations for the inorganic carbon system that you wrote in (a) to obtain two expressions for [HCO3-] and [CO32-] as a function of pCO2 and [H+] only. (Show your steps.) [2 Points] (photo attached for hint)
(c) Based on the equations you found, if pH was to decrease, while pCO2 remained the same, how would [HCO3-] and [CO32-] change in seawater? Which one would show the greater change? (Explain you reasoning.) [2 Points]
Answers
(a) The main inorganic carbon system reactions in seawater involve the transfer of CO2 from the air phase into the water phase. The associated chemical equilibrium equations are as follows:
CO2 (aq) + H2O ↔ H2CO3 ↔ HCO3- + H+ ↔ CO32- + 2H+
CO2 (aq) is the dissolved CO2 in the water phase, H2CO3 is the carbonic acid, HCO3- is the bicarbonate ion, H+ is the hydrogen ion, and CO32- is the carbonate ion.
(b) Manipulation of the chemical equilibrium equations yields the following expressions for [HCO3-] and [CO32-] as a function of pCO2 and [H+] only:
[HCO3-] = K1*pCO2/[H+]
[CO32-] = K2*K1*pCO2/[H+]2
Where K1 and K2 are the carbonic acid and bicarbonate ion dissociation constants, respectively.
(c) If pH were to decrease while pCO2 remained the same, [HCO3-] would decrease more than [CO32-]. This is because the equation for [HCO3-] is directly proportional to [H+], whereas the equation for [CO32-] is inversely proportional to [H+]. As pH decreases, [H+] increases, so [HCO3-] decreases more drastically than [CO32-].
Know more about inorganic carbon here
https://brainly.com/question/2169750#
#SPJ11
Consider the following set of compounds: CFCl3, CHFCl2, CF3Cl, and CHF3. Assuming that equal numbers of moles of each were released into the air at ground level, rank these four compounds in terms of their potential to catalytically destroy ozone in the stratosphere. Explain your ranking.
Answers
Ranking the compounds in terms of their potential to catalytically destroy ozone in the stratosphere from highest to lowest:
CFCl3 (CFC-11)
CHFCl2 (HCFC-123)
CF3Cl (CFC-13)
CHF3 (HFC-23)
Ozone destruction in the stratosphere is primarily driven by the presence of halogen-containing compounds, such as chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). These compounds contain chlorine (Cl) and/or fluorine (F), which can act as catalysts in the destruction of ozone.
CFCl3 (CFC-11):
This compound contains three chlorine atoms and no fluorine atoms. Chlorine is highly reactive with ozone and acts as an efficient catalyst in the destruction of ozone. CFCl3 is one of the most potent ozone-depleting substances, with a high ozone depletion potential (ODP) value. It remains in the atmosphere for a long time, allowing its catalytic effect on ozone destruction to persist.
CHFCl2 (HCFC-123):
This compound contains one chlorine atom and one fluorine atom. HCFCs are considered transitional substances between CFCs and hydrofluorocarbons (HFCs) due to their lower ODP values. While HCFC-123 has a lower ODP compared to CFCl3, it can still contribute to ozone depletion as the chlorine atom can catalytically destroy ozone. However, its impact is lower due to the presence of the fluorine atom, which makes it less reactive towards ozone.
CF3Cl (CFC-13):
This compound contains three fluorine atoms and one chlorine atom. Fluorinated compounds generally have a lower ODP compared to chlorinated compounds as fluorine is less reactive towards ozone. While CF3Cl does contain a chlorine atom, the presence of three fluorine atoms makes it less effective as a catalyst for ozone destruction compared to CFCl3.
CHF3 (HFC-23):
This compound contains three fluorine atoms and no chlorine atoms. HFCs are non-ozone depleting compounds and do not contribute to the destruction of stratospheric ozone. CHF3, commonly known as HFC-23, does not have any catalytic effect on ozone destruction due to the absence of chlorine. It is considered a greenhouse gas with a high global warming potential (GWP), but it does not directly impact ozone depletion.
In summary, CFCl3 (CFC-11) has the highest potential to catalytically destroy ozone in the stratosphere, followed by CHFCl2 (HCFC-123), CF3Cl (CFC-13), and CHF3 (HFC-23). The presence of chlorine atoms in the compounds increases their ozone-depleting potential, while the presence of fluorine atoms reduces their reactivity towards ozone. It is important to note that the production and use of ozone-depleting substances, such as CFCs and HCFCs, have been phased out under international agreements like the Montreal Protocol to protect the ozone layer.
To know more about stratosphere visit:
https://brainly.com/question/30942738
#SPJ11
Which best symbolizes the hydrogen bonding between two water molecules? group of answer choices
Answers
Answer:
here go your answer

An angled dashed line (---->) is the ideal way to depict the hydrogen bonding between two water molecules.
Hydrogen bonding is the process by which an electronegative atom in a separate molecule is pulled to a hydrogen atom that is linked to a strongly electronegative atom, such as nitrogen, oxygen, or fluorine. It is a unique sort of bonding because hydrogen and the electronegative atom have very different electronegativities. Hydrogen bonds are responsible for a wide range of important traits and occurrences. One of its most notable effects is on a substance's physical properties. Compounds have high melting and boiling points because the hydrogen bonds must be broken in order for a substance to change states. An angled dashed line (---->) is the ideal way to depict the hydrogen bonding between two water molecules.
To know more about hydrogen bonding, here:
https://brainly.com/question/31139478
#SPJ6
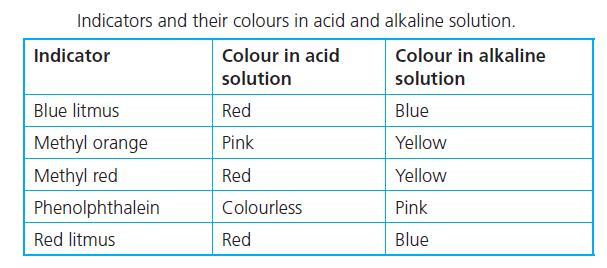
help i suck at chemistry

Answers
Answer:
1. Acid - Red
2. Base - Yellow
3. Salt - Yellow if the reaction produces a base
Explanation:
In an acidic medium, methyl orange turns red, while in a basic medium, it turns yellow.
Sodium chloride solution produces sodium hydroxide, NaOH which is a strong base. Using methyl orange as an indicator gives a yellow colour solution for NaOH.
There are acidic, neutral, and basic salts. Sodium chloride (NaCl) produces a base therefore it would turn yellow as well but likely less distinct than the base.
Answer:
Hello methyl orange is a pH indicator that is commonly used.
If you drip methyl orange to an acidic liquid it will give you the color red.
If it turns yellow after you drip it then the liquid should be a base.
And it gives a yellowish color for neutral liquids
But in this case salt (NaOH) has an exceptional situation which turns orange after adding m.o.
There is no logical explanation (at least for high school level) I am afraid that you need to memorize it.
This chard attached below may help you to recognize it
good luck, hope it helped<3!
What is the overall charge of an ion that has 11 protons, 13 electrons and 12 neutrons?
Answers
Answer:
+1
Explanation:
To calculate the charge on an ion, we simply use the number of protons and electrons. Protons are the positively charged subatomic particles. The electrons are the negatively charged particles in an atom.
When the number of protons are greater than that of electrons, the ion is positively charged.
When the number of protons are lesser than that of electrons, the ion is negatively charged.
When the number of protons and electrons are equal, the ion is neutral.
Answer Below and top
Charge = Protons - Electrons = 11 - 10 = +1
Have a good day and plz mark me brainliest!!
Answer:
-2
Explanation:
9. (05.06 mc)
during an experiment, the percent yield of calcium chloride from a reaction was 80.34%. theoretically, the expected amount should have been 115 grams. what was the actual yield
from this reaction?
caco, + hc - cacl + co, + h20 (5 points)
a. 090.1 grams
b. 92.4 grams
c. 109.2 grams
d. 115.3 grams
Answers
The actual yield of the experiment given the data from the question is 92.4 g (Option B)
What is percentage yield?This is the ratio of the actual yield to the theoretical yield of a given reaction multiplied by 100
Percentage yield = (Actual / Theoretical) × 100
What is actual yield?This is the amount obtained from the expriment procedures
What is theoretical yield?This is the amount obtained from simple calculations as regarding the experiment.
How to determine the actual yieldPercentage yield = 80.34%Theoretical yield = 115 gActual yield =?Actual yield = percentage yield × theoretical yield
Actual yield = 80.34% × 115
Actual yield = 0.8034 × 115
Actual yield = 92.4 g
Learn more about stoichiometry:
https://brainly.com/question/14735801
#SPJ1