A collection of these atoms is kept very cold, so that all are in the ground state. Light consisting of photons with a range of energies from 1 to 7. 5 ev passes through this collection of atoms. What photon energies will be absorbed?.
Answers
Only photons with energies equal to the difference between the ground state energy and higher energy states are absorbed.
The process of absorption of photons occurs when the energy of the incident photons equals the energy difference between the ground state and any higher energy state. This concept is defined by the equation: ΔE = hν, where ΔE is the energy difference between two energy states, h is Planck's constant, and ν is the frequency of the incident photons. So, the photon energies that will be absorbed are the ones that have energies equal to the difference between the ground state energy and higher energy states.
Therefore, in a collection of atoms that are in the ground state and exposed to photons with a range of energies from 1 to 7.5 eV, only photons with energies equal to the difference between the ground state energy and higher energy states will be absorbed.
To know more about absorption of photons, click here
https://brainly.com/question/32261783
#SPJ11
Related Questions
What kind of energy can be transferred?
Help quick please.
Answers
Answer:
Any kind, as long as there is an action.
Answer:
Energy can be transferred from one form to another like kinetic energy to potential energy, light energy to heat energy, kinetic energy to electrical energy, light energy to chemical energy .etc ...
Explanation:
brainest please
3Li HPO
How many elements?
How many Atoms of Lithium (Li)?
How many Atoms of Hydrogen (H)?
How many Atoms of Phosphorus (P)?
How many Atoms of Oxygen (0)?
How many total Atoms in this Chemical Formula?
1 2 3 4 5
Answers
Answer:
1) There are Four Elements
Li , H , P , O
2) Li have 3 atoms.
3) H have 3 atoms.
4) P have 3 atoms.
5) O have 3 atoms.
Hey besties can y'all help me out
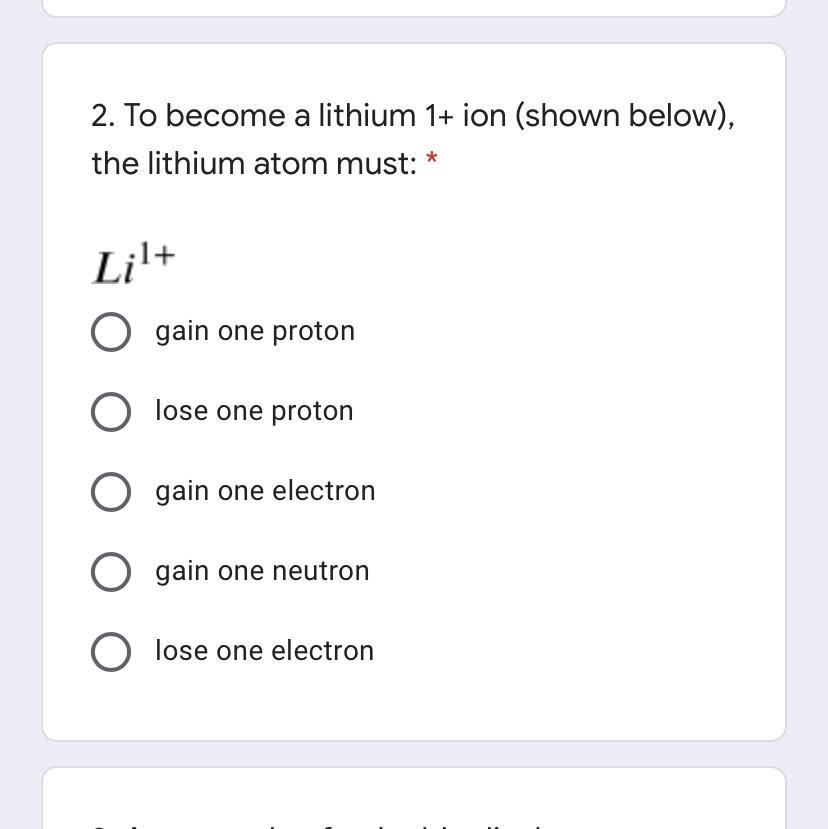
Answers
you need to lose one electron
A teacher has given a group assignment to classify certain things as matter or energy. Most of the students in the group have decided on the following classification:
Matter: atoms, sugar, water, air
Energy: heat, potential, kinetic, sound
However, there is one student who is arguing that all of the things listed in both categories can be classified as energy, and so that must mean that they can all be classified as matter, too.
Which of the following provides the best scientific explanation as to why the things classified as energy are not also classified as matter?
Answers
The things classified as energy can not be classified as matter based on the description of both.
Energy is just the ability or the capacity to get work done. Energy in itself is massless, it is just a property of a body, a system or an object.Matter refers to any entity that has mass and occupy space. A matter can be a solid, liquid, or even a gas.From these definitions, it is clear that the things classified as energy can not also be classified as matter. Energy is just a property or a concept.
More on matter and energy can be found here: https://brainly.com/question/23490755
Choose the correct products for the double replacement reaction below. Click here to access the solubility rules to determine which product, if any, forms a solid precipitate in the reaction. AgNO3 KCl Upper K l Upper Pb (Upper N Upper O Subscript 3) Subscript 2 Baseline right arrow. ? K O2 NCl KNO3 AgCl AgK ClNO3 K2NO3 AgCl2.
Answers
In both cases, the precipitates were AgCl and PbI2 respectively.
What is a precipitate?A precipitate is a solid product obtained from the reaction of two aqueous phase species. Let us now consider the two reactions listed in the question;
AgNO3(aq) + KCl(aq) ----> AgCl(s) + KNO3(aq)2KI(aq) + Pb(NO3)2 (aq) ----->PbI2(s) + 2KNO3(aq)We can see that in both cases, the precipitates were AgCl and PbI2 respectively.
Learn more about precipitates: https://brainly.com/question/24846690
Answer:
B and B
Explanation:
edge 2022
ten joules of heat energy are transferred to a sample of ideal gas at constant volume. as a result, the internal energy of the gas question 9 options: increases by 10 j increases by less than 10 j increases by more than 10 j remains unchanged
Answers
Ten Joules of heat energy are transferred to a sample of ideal gas at constant volume. As a result, the internal energy of the gas increases by 10 Joules.
Calculation:
dU= change in internal energy Q=heat absorbed by the system W=work done by the system dU = W+Q If we add 10 Joules of heat, and considering that the volume doesn't change. It is a isobaric process in which -- No Work Is Done -- then it would increase by 10 Joules.W= 0, Q = 10. Therefore, dU = W+Q.so the answer is dU = 0+10. The internal energy of the gas increases by 10 Joules.To learn more how the internal energy of the gas is calculated refer to:
https://brainly.com/question/29574181
#SPJ4
calculate the temperature (in k) at which 0.0938 mol of o2 has a pressure of 740 torr in a 1.0l container.
Answers
The temperature (in k) will be 126.509 K
Number of moles of Oxygen = 0.0938 mol
Pressure = 740 torr or 98658.6 Pa
Volume = 1.0 L or 0.001 m3
Temperature in K = ?
To find out the temperature (T) we use the ideal gas equation
PV = nRT
As we have to find the temperature so rearrange the equation for T
T = PV / nR
value of R = 8.314 J/mol.K
Put the values in the equation
V = 98658.6 * 0.001 / 0.0938 * 8.314
V = 0.000974 / 0.779853
V = 98.6596 / 0.779853
V = 126.509 K
You can also learn about the ideal gas equation from the following question:
https://brainly.com/question/3637553
#SPJ4
Classify each of the following as a physical or chemical change. For any chemical change list at least one clue to support your answer. a. A copper wire is bent. b. Charcoal burn in a grill. c. Bread dough rises when yeast is added. d. Sugar dissolves in water.
Answers
Answer:
a. physical
b.chemical
c.chemical
d.physical
Explanation:
A. You can easily unbend the wire and reverse the change
D. No new products were created
THE ANSWER IS B AND C AS CHEMICAL CHANGES
Answer:thank you
Explanation:because I’m lazy
Earth’s outermost layer is separated into a dozen or more large and small slabs, called _______. A. continental crust B. tectonic plates C. granite slabs D. Pangea
Answers
Answer:
Tectonic Plates
Explanation:
Answer:
Tectonic plates
Explanation:
4. How many weeks are equal to 2.94 x 10 to the power of 6 minutes?
Answers
Answer:
292 weeks
Explanation:

The answer would be 291.67 weeks are equal to 2.94 x 10 to the power of 6 minutes.
What are Arithmetic operations?Arithmetic operations can also be specified by subtracting, dividing, and multiplying built-in functions. The operator that performs the arithmetic operation is called the arithmetic operator.
How to Convert Minutes to Weeks?Divide the time by the conversion ratio to convert a minute measurement to a week measurement.
You can use this simplest technique to translate because one week is equivalent to 10,080 minutes:
weeks = minutes ÷ 10,080
The time in weeks is equal to the minutes divided by 10,080.
We have to determine the number of weeks is equal to 2.94 x 10 to the power of 6 minutes.
So the number of weeks = 2.94 × 10⁶ minutes / 10,080
= 291.67 weeks
Therefore, 291.67 weeks are equal to 2.94 x 10 to the power of 6 minutes.
Learn more about Arithmetic operations here:
brainly.com/question/25834626
#SPJ2
If a plant cell is placed in a solution and the cell shrivels up, what type of solution was it placed in? How do you know?
Answers
Answer:
The cell is in a Hypotonic solution. When a cell is in a Hypotonic solution the lower osmotic pressure causing water to move across the pressure gradient into the solution causing the cell to shrivel.
an overhead fan is turned on what energy is used for the fan
Answers
Which of the following peptides results after a single Edman degradation of the tetrapeptide Gly-Phe-Tyr- Ser? a. Gly-Phe b. Gly-Phe-Tyr c. Tyr-Ser d. none of these e. Phe-Tyr-Ser
Answers
After a single Edman degradation of the tetrapeptide Gly-Phe-Tyr-Ser, the resulting peptide would be "Phe-Tyr-Ser" (option e).
The process of Edman degradation involves selectively removing one amino acid at a time from the N-terminus of a peptide chain. In this case, the tetrapeptide Gly-Phe-Tyr-Ser undergoes a single Edman degradation.
During the Edman degradation, the N-terminal amino acid (Gly) is cleaved off and identified. The resulting peptide after this degradation step would be the remaining amino acids.
Learn more about Edman degradation on:
https://brainly.com/question/24317649
#SPJ11
An unknown amount of Al203 decomposed producing 215 g of solid aluminum. 2Al2O3=4Al+3O2 How many grams of oxygen gas should be produced
Answers
Answer:
191.11 grams of oxygen gas should be produced.
Explanation:
The balanced reaction is:
2 Al₂O₃ → 4 Al + 3 O₂
By stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Al₂O₃: 2 molesAl: 4 molesO₂: 3 molesBeing the molar mass of each compound:
Al₂O₃: 102 g/moleAl: 27 g/moleO₂: 32 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Al₂O₃: 2 moles* 102 g/mole= 204 gramsAl: 4 moles* 27 g/mole= 108 gramsO₂: 3 moles* 32 g/mole= 96 gramsThen you can apply the following rule of three: if by stoichiometry 108 grams of aluminum are produced along with 96 grams of oxygen, 215 grams of aluminum are produced along with how much mass of oxygen?
\(mass of oxygen=\frac{215 grams of aluminum*96 grams of oxygen}{108grams of aluminum}\)
mass of oxygen= 191.11 grams
191.11 grams of oxygen gas should be produced.
write a balanced net ionic equation for the reaction of nibr2(aq) with (nh4)2s(aq).
Answers
The balanced net ionic equation for the reaction of NiBr2(aq) with (NH4)2S(aq) is:
Ni2+(aq) + S2-(aq) → NiS(s)
Note that the spectator ions NH4+ and Br- do not participate in the reaction and are not included in the net ionic equation.
Spectator ions are ions that do not participate in a chemical reaction, meaning they do not undergo any chemical change during the reaction. They are present in both the reactants and the products and do not affect the outcome of the reaction.
Spectator ions can be identified by looking at the balanced chemical equation of the reaction and canceling out ions that appear on both sides of the equation.
Visit here to learn more about spectator ions brainly.com/question/28913274
#SPJ11
The balanced net ionic equation for the reaction of NiBr2(aq) with (NH4)2S(aq) is:
Ni2+(aq) + S2-(aq) → NiS(s)
Note that the spectator ions NH4+ and Br- do not participate in the reaction and are not included in the net ionic equation.
Spectator ions are ions that do not participate in a chemical reaction, meaning they do not undergo any chemical change during the reaction. They are present in both the reactants and the products and do not affect the outcome of the reaction.
Spectator ions can be identified by looking at the balanced chemical equation of the reaction and canceling out ions that appear on both sides of the equation.
Visit here to learn more about spectator ions brainly.com/question/28913274
#SPJ11
a person suffering from beriberi (deficiency of vitamin b1) lacks the ability to readily produce
Answers
A person suffering from beriberi (deficiency of vitamin B1) lacks the ability to readily produce energy. Beriberi is a disease that is caused due to the deficiency of vitamin B1 (thiamine) in the body.
Thiamine is a water-soluble vitamin and is an essential nutrient that is required for the breakdown of carbohydrates into glucose to produce energy. A deficiency of vitamin B1 can affect the nervous system, heart, and muscles in the body, leading to the symptoms of beriberi.
Beriberi is more common in people who consume a diet that is low in thiamine, such as those who consume a diet high in polished rice, alcoholics, and people with gastrointestinal disorders.
Symptoms of beriberi include muscle wasting, tingling and numbness in the limbs, confusion, difficulty walking, and heart failure. In summary, a person suffering from beriberi (deficiency of vitamin B1) lacks the ability to readily produce energy.
To know more about beriberi, refer here:
https://brainly.com/question/30517525#
#SPJ11
Earth's gravitational potential energy: GPE = mgh = Gravity (9.81m/s2) *
Mass (kg) x Height (m)
Kinetic energy: KE= Imov?
How do the mass and speed of an object affect the kinetic energy?
Answers
Answer:
as mass and the square of speed is directly proportional to the the kinetic enegry so, more there will be mass and speed there will be more kinetic enegry.
Help pleasee I need help to write the formulas i’m so lost

Answers
Answer:
NH4
Explanation:
Which of the following are changes that might take place during a chemical reaction? Select all that apply Choices are: - bonds are formed; - the reactants gain mass; - and Aton loses a proton from its nucleus; -a neutron is converted into a proton; -a proton is added to the nucleus of a atom; -an atom acquired an electron lone pair
Answers
-Bonds are formed- When atoms collide, they may form bonds with one another. This happens when the electrons in the outermost energy level of each atom interact and form a shared pair of electrons. The shared electrons hold the atoms together in a chemical bond.
The reactants gain massA chemical reaction involves the rearrangement of atoms. When the atoms are rearranged, they form new bonds with one another. As a result, the overall mass of the reactants increases.
And Aton loses a proton from its nucleusIn some reactions, an atom may lose a proton from its nucleus. This can happen when the atom reacts with another atom that has a stronger affinity for the proton.
A neutron is converted into a protonIn some reactions, a neutron may be converted into a proton. This can happen when the neutron reacts with another particle that has a higher energy than the neutron.
A proton is added to the nucleus of a atomIn some reactions, a proton may be added to the nucleus of an atom. This can happen when the atom reacts with another atom that has a weaker affinity for the proton.
An atom acquired an electron lone pairIn some reactions, an atom may acquire an electron lone pair. This can happen when the atom reacts with another atom that has a higher energy than the atom.
The products have less mass than the reactantsIn some reactions, the products have less mass than the reactants. This can happen when the atoms in the reactants are rearranged to form new bonds with one another.
Learn more about atoms:
https://brainly.com/question/6258301
#SPJ4
cis,cis-hexa-2,4-diene draw the molecule on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars.
Answers
Hexa-2,4-diene-1,6-diol,cis,cis- Muconic alcohol is the name of this Molecule.
What structure does 2-nitrophenol have?The chemical compound 2,4-Dinitrophenol (often known as 2,4-DNP or simply DNP) has the formula HOC6H3(NO2)2. It smells pleasant and musty and is a yellow, crystalline substance. It is soluble in the majority of organic solvents as well as aqueous alkaline solutions, sublimates, and is volatile with steam.
What is henna, 2 amino-4,6 dinitrophenol?Picric acid is a base that can be made into picramic acid, also known as 2-amino-4,6-dinitrophenol, by neutralizing it with an alcoholic solution of ammonium hydroxide. The resulting solution is then treated with hydrogen sulfide, which causes it to turn red and produce sulfur and red crystals.
To know more about Molecule visit:-
https://brainly.com/question/19556990
#SPJ4
What is the boiling point elevation of a solution prepared by dissolving 0.500 mol of sugar in 0.750 kg of water?0.3410.7681.24792
Answers
1) List known data.
Moles of sugar: 0.500
Mass of water: 0.750 kg
2) List unknown data.
Boiling point elevation:
3) Set the equation for boiling point elevation
\(\Delta T=K_bm\)ΔT: change in temperature
Kb: Ebulloscopic constant (0.512 ºC/molal).
m: molality.
4) Molality of the solution
\(m=\frac{\text{moles of solute}}{\text{kilograms of solvent}}\)\(m=\frac{0.500\text{ mol of sugar}}{0.750\text{ kg of water}}=0.6667m\)5) Plug in values in the equation
\(\Delta T=\frac{0.512ºC}{\text{molal}}\cdot0.6667\text{ molal=0.3413ºC}\)The change in the boiling point is 0.341ºC.
NO LINKS!!!!!!!!
how many joules are needed to heat 20g of Cu from 10 to 50 degrees C?
Answers
Answer:
q=309.6 J
Explanation:
Here we'll be using the formula of specific heat capacity: Q=mcΔT
Q= heat (joules)
m=mass (g)
c=specific heat capacity
ΔT= change in temperature > Final temp. - initial temp.
Let's plug in what we know and solve for q=heat.
q=(20)(0.387)(50-10)
q=(20)(0.387)(40)
q=309.6 joules
Depending on your teacher and the problem, they might want you to keep the temperature in C. Mine wanted them all in Kelvin, so if we were to convert it to Kelvin it would be:
273 + 40= 313 K
q=(20)(0.387)(313)
q=2422.6 J
At what temperature (in oC) does 13.09 g of argon occupy a volume of 9.82 L at a pressure of 1.47 atm
Answers
A temperature of -80.2°C, 13.09 g of argon occupies a volume of 9.82 L at a pressure of 1.47 atm.
To solve this problem, we can use the ideal gas law, which relates the pressure, volume, and temperature of a gas to the number of moles of gas present:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
We can rearrange this equation to solve for the temperature:
T = PV/nR
First, we need to calculate the number of moles of argon present:
n = m/M
where m is the mass of argon and M is the molar mass of argon. The molar mass of argon is approximately 39.95 g/mol.
n = 13.09 g / 39.95 g/mol = 0.3272 mol
Next, we can substitute the given values into the ideal gas law and solve for the temperature:
T = (1.47 atm) x (9.82 L) / (0.3272 mol x 0.08206 L•atm/mol•K)
T = 193 K
Finally, we can convert the temperature from Kelvin to Celsius:
T(°C) = T(K) - 273.15
T(°C) = -80.2°C
Therefore, at a temperature of -80.2°C, 13.09 g of argon occupies a volume of 9.82 L at a pressure of 1.47 atm.
To know about the ideal gas law,
https://brainly.com/question/28257995
#SPJ4
Which statement accurately describes valence electrons?

Answers
the water in a beaker has a volume of 50 millimeters, is this an extensive property?
Answers
No, the volume of water in a beaker is not an extensive property.
Extensive properties are those that depend on the amount or size of the substance being measured. In other words, they are properties that change with the quantity of the substance. Examples of extensive properties include mass, volume, and total energy.
In the given scenario, the volume of water in the beaker is 50 milliliters. This volume remains the same regardless of the quantity of water present. Whether it's 50 milliliters or 500 milliliters, the volume measurement does not change. Therefore, the volume of water in the beaker is an example of an intensive property.
Intensive properties are independent of the amount or size of the substance. They are characteristics that remain constant regardless of the quantity of the substance. Examples of intensive properties include temperature, density, and color.
It's important to note that the distinction between extensive and intensive properties depends on the specific property being considered. While volume is typically an extensive property for a bulk substance, in the case of a fixed volume of water in a beaker, it becomes an intensive property.
In summary, the volume of water in a beaker is not an extensive property but rather an intensive property because it does not change with the quantity of the substance.
For more such questions on extensive property visit:
https://brainly.com/question/13055036
#SPJ8
If 2 carbon atoms, underwent fusion. What element would you get?
Answers
Answer:
Two Helium atoms
If two carbon atoms underwent fusion (of 600 million kelvin) you would get two helium atoms.
Which shows an isomer of the molecule below?

Answers
Answer: C
Explanation:
Don’t trust the other person it’s not A
*view photo for answer*
pay attention! the answer choices aren't always in the same order!

3. When a new substance is formed with different properties than the original substance it
is called a
A. Chemical change
B. Physical change
C. Freezing
D. Boiling
Answers
Answer:
A) Chemical Change
Explanation:
Chemical changes occur when bonds are broken and formed between molecules or atoms. This means that one substance with a particular set of properties (such as melting point, color, taste, etc.) is turned into a different substance with different properties.
A 2.0 l container of oxygen had a pressure of 3.2 atm. what volume would be necessary to decrease the pressure to 1.0 atm? use the formula: p1v1 = p2v2 0.625 l 0.64 l 6.4 l 64 l
Answers
The volume that would be necessary to decrease the pressure to 1.0 atm is mathematically given as
v2=6.4l
What volume would be necessary to decrease the pressure to 1.0 atm?Question Parameter(s):
A 2.0 l container of oxygen had a pressure of 3.2 atm
Generally, the equation for the is mathematically given as
P1v1=p2v2
Therefore
v2=piv1/p2
v2=2*3.2/1
v2=6.4l
In conclusion, the volume is
v2=6.4l
Read more about volume
https://brainly.com/question/1578538
#SPJ4
Answer:
CExplanation:
which lewis electron-dot diagram represents the bonding in potassium iodide
Answers
The lewis electron-dot diagram which represents the bonding in potassium iodide, KI is give in image attached.
The correct answer choice is option 1.
What is meant by lewis electron-dot diagram?The lewis structure can simply be defined as those diagrammatic chemical representations which describes the bonding between atoms in a particular molecular structure of a chemical substance. From the context of the above given task, the structure is bonded by eight different electrons.
The importance of lewis electron-dot structures cannot be overemphasized. Some of its significances are as follows:
It helps to us understand how chemical substances are bonded or how they bond.It also shows how electrons are arranged It gives us an indepth knowledge of arrangement of valence electrons of a molecule.In conclusion, we can now confirm from the explanation given above that potassium iodide is solid chemical compound.
The complete image of the options of the question is also attached.
Read more on lewis structure:
https://brainly.com/question/13820123
#SPJ1

