A drum used to transport crude oil has a volume of 162 L. How many grams of water, as steam, are required to fill the drum at 1. 00 atm and 1069°C? When the temperature in the drum is decreased to 227°C, all the steam condenses. How many mL of water (d = 1. 00 g/mL) can be collected?
Answers
When the steam condenses, we can collect 204.06 mL of water.
To answer this question, we need to use the ideal gas law equation, PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
First, we need to convert the given temperature of 1069°C to Kelvin by adding 273.15, giving us 1342.15 K. We can then calculate the number of moles of steam needed to fill the drum by rearranging the ideal gas law equation to solve for n: n = PV/RT.
Plugging in the given values, we get n = (1.00 atm)(162 L)/(0.08206 L·atm/mol·K)(1342.15 K) = 11.32 moles of steam.
To calculate the mass of water in grams, we can use the fact that 1 mole of water weighs 18.015 g. Thus, the mass of water needed to fill the drum as steam is 11.32 moles x 18.015 g/mol = 204.06 g.
When the temperature in the drum is decreased to 227°C, all the steam condenses back into water. The heat released by the steam is given off to the surroundings, and the water vapor loses energy and condenses to form liquid water. We can calculate the volume of water that is formed using the fact that 1 mL of water has a mass of 1.00 g.
Thus, the mass of the water that forms is 204.06 g, which is equivalent to 204.06 mL of water. Therefore, when the steam condenses, we can collect 204.06 mL of water.
To know more about steam, visit:
https://brainly.com/question/15447025#
#SPJ11
Related Questions
Organisms typically have more than one form of each gene. If one form can mask the appearance of another form, that form is considered _______ the other form.
A.
better than
B.
dominant over
C.
recessive to
D.
worse than
Answers
If one form of a gene can mask the appearance of another form, that form is considered dominant over the other form. Option B.
What are dominant alleles?According to Mendel, genes are usually made up of 2 alleles. These alleles can be the same or different. When the alleles are the same, the gene is said to be homozygous. If the alleles are different, the gene is said to be heterozygous.
When the two alleles that make up a gene are different, one will be dominant and the other will be recessive. The dominant gene masks the effect of the recessive gene. In other words, the recessive gene cannot be expressed as long as it coexists with the dominant gene. In order for it to be expressed, it has to be in two copies or a homozygous recessive form.
For the dominant allele, however, only one copy is needed for it to be expressed.
In summary, if one form of a gene can mask the appearance of another form, that form is considered dominant over the other form.
More on genes can be found here: https://brainly.com/question/5519888
#SPJ1
A 2.00L flask was filled with 4.00 mol of HI at a certain temperature and given sufficient time to react. At equilibrium the concentration of H2 was 0.400 M. Find the equilibrium concentrations of I2 and HI and then find the Keq at this temperature.
Answers
Answer:
The equilibrium concentration of I₂ is 0.400 M and HI is 1.20 M, the Keq will be 0.112.
Explanation:
Based on the given information, the equilibrium reaction will be,
2HI (g) ⇔ H₂ (g) + I₂ (g)
It is given that 4.00 mol of HI was filled in a flask of 2.00 L, thus, the concentration of HI will be,
= 4.00 mol/2.00 L
= 2.00 mol/L
Based on the reaction, the initial concentration of 2HI is 2.00, H₂ is 0 and I₂ is O. The change in the concentration of 2HI is -x, H₂ is x and I₂ is x. The equilibrium concentration of 2HI will be 0.200-x, H₂ is x and I₂ is x.
It is given that at equilibrium, the concentration of H₂ or x is 0.400 M.
Now the equilibrium concentration of HI will be,
= 2.00 -2x
= 2.00 - 2 × 0.400
= 1.20 M
The equilibrium concentration of I₂ will be,
I₂ = x
= 0.400 M
The equilibrium constant (Keq) will be,
Keq = [H₂] [I₂] / [HI]²
= (0.400) (0.400) / (1.20)²
= 0.112
Thus, the Keq of the reaction will be 0.112.
what happens in terms of kinetic energy and the movement of particles during transition shifts
Answers
During transition shifts, the kinetic energy and movement of particles undergo significant changes. Transition shifts refer to phase transitions, such as melting, freezing, vaporization, condensation, and sublimation, where a substance changes from one phase to another.
As a substance undergoes a transition shift, there are changes in the average kinetic energy and movement of its particles. Let's consider the example of water transitioning from a solid (ice) to a liquid (water) during melting.
During melting, the temperature of the ice increases, imparting energy to the ice particles. As a result, the average kinetic energy of the particles increases. The particles gain enough energy to overcome the intermolecular forces holding them in a fixed arrangement, causing them to break free from their ordered positions.
As the transition progresses, the particles gain enough energy to move more freely and with greater speed. The increased kinetic energy allows the particles to break the bonds between them and move past each other, resulting in the transition from a solid to a liquid state.
Similarly, during other phase transitions, the average kinetic energy and movement of particles change accordingly. For example, during vaporization (transition from liquid to gas), particles gain even more energy, causing them to move rapidly and independently in the gas phase.
Overall, during transition shifts, there is an increase in the average kinetic energy of particles, enabling them to break intermolecular bonds and move more freely as they transition between different phases.
For more such questions on kinetic energy visit;
https://brainly.com/question/7694005
#SPJ8
What mass of carbon dioxide (co2) can be produced from 11.2 g of c6h14 and excess oxygen?
Answers
The mass of carbon dioxide produced in the complete combustion of hexane is 34.33 g.
C6H14 or hexane, with a molar mass of 86.18 g/mol, is reacted with excess oxygen to form carbon dioxide and water. The balanced combustion reaction is shown below.
2C6H14 + 19O2 → 12CO2 + 14H2O
The limiting reactant, hexane, would be the basis of the produced carbon dioxide. First, 11.2 g of hexane is converted to mol of hexane by dividing it by its molar mass.
Mol hexane= 11.2 g / 86.18 g/mol
Mol hexane= 0.13 mol
Then, convert mol of hexane to mol of carbon dioxide through the use of a stoichiometric ratio. Burning 2 mol of hexane produces 12 mol of carbon dioxide as can be seen in the reaction.
Mol co2= 0.13 mol* (12 mol carbon dioxide/2 mol hexane)
Mol co2= 0.78 mol
Lastly, convert mol of co2 to the mass of co2 by multiplying it with its molar mass, 44.01 g/mol.
Mass co2= 0.78 mol*44.01 g/mol
Mass co2= 34.33 g
For more information regarding combustion, please refer to the link https://brainly.com/question/23992512.
#SPJ4
give three simple examples of physical separations of mixtures that one might do in the home. for each, give the mixture, the physical property exploited, and the separation method (if applicable).
Answers
Three simple examples of physical separations of mixtures that can be done at home include filtering a mixture of sand and water to separate the solid particles, using evaporation to separate a solution of saltwater, and using magnetism to separate a mixture of iron filings and sand.
In the first example, a mixture of sand and water can be separated by exploiting the physical property of particle size. Since sand particles are larger and insoluble in water, they can be separated by filtration. By pouring the mixture through a filter paper or a fine sieve, the sand particles are retained while the water passes through, resulting in the separation of the two components. In the second example, a solution of saltwater can be separated by utilizing the physical property of boiling point. Through the process of evaporation, the water in the solution can be vaporized, leaving behind the salt crystals. This separation method is based on the fact that water has a lower boiling point compared to salt, allowing the water to change into a gas while the salt remains solid.
In the third example, a mixture of iron filings and sand can be separated by exploiting the physical property of magnetism. Iron is magnetic, whereas sand is not. By using a magnet, the iron filings can be attracted and separated from the sand. The magnet is brought close to the mixture, and the iron filings cling to it, allowing for the removal of the magnetic component from the non-magnetic sand. This separation method takes advantage of the different physical properties of the two components to achieve the separation.
To learn more about mixtures refer:
https://brainly.com/question/24647756
#SPJ11
which statement about co2 is false? question 11 options: more co2 dissolves in the blood plasma than is carried in the rbcs. co2 concentrations are greater in venous blood than arterial blood. its accumulation in the blood is associated with a decrease in ph. its concentration in the blood is decreased by hyperventilation.
Answers
The statement more CO₂ dissolves in the blood plasma than is carried in the RBCs is FALSE
CO₂ is produced as a waste product during cellular respiration, and it must be transported from the cells to the lungs for exhalation. In the blood, most of the CO₂ is transported in the form of bicarbonate ions (HCO₃-), which are produced when CO₂ reacts with water (H₂O) in the presence of the enzyme carbonic anhydrase.
This reaction occurs mainly inside the red blood cells (RBCs), where the enzyme is most abundant. The HCO₃- ions are then transported in the plasma, while some of the CO₂ also remains dissolved in the plasma.
Additionally, CO₂ concentrations are greater in venous blood than arterial blood, and its accumulation in the blood is associated with a decrease in pH due to the formation of carbonic acid (H₂CO₃). This decrease in pH can lead to acidosis and other health issues. Furthermore, hyperventilation decreases the concentration of CO₂ in the blood by increasing the rate of exhalation, which can be helpful in certain situations such as in treating respiratory acidosis.
To know more about blood plasma, refer here:
https://brainly.com/question/30788908#
#SPJ11
4. NH4Cl
What is the compound?
Answers
Answer:
ammonium chloride
Explanation:
Because NH is ammonium and Cl is chlorine
A 10 month old with heart failure weighs 10 kg. Digoxin is prescribed as 10mCg/gg/day to be given every 12 hours. How much is given for each dose? 11. Penicillin is given to a 2 year old prior to dental work. The child weighs 44 los, The order is for 25mg/kg to be given 2 hours before the procedure. The penicilin comes in 250πd5 mL. How much of the medication will the nurse administer
Answers
1 ) The dose of Digoxin to be administered is 50 mcg per dose 2) To achieve a dose of 500 mg, the nurse will need to administer 10 mL of the medication.
1) Digoxin is prescribed for a 10-month-old with heart failure who weighs 10 kg at a dose of 10 mcg/kg/day to be given every 12 hours. Determine how much is given per dose Digoxin dose = 10 mcg/kg/dayWeight of the patient = 10 kgTotal dosage = (10 mcg/kg/day) × 10 kg = 100 mcg/day
Digoxin to be given every 12 hours: 100 mcg/day ÷ 2 = 50 mcg/doseTherefore, the dose of Digoxin to be administered is 50 mcg per dose.2) Penicillin is given to a 2-year-old before dental work. The child's weight is 44 lbs. The order is for 25 mg/kg to be given two hours before the procedure. '
The penicillin comes in 250 mg/5 mL. How much of the medication will the nurse administer Order: 25 mg/kg Weight of the patient: 44 lbs Convert pounds to kilograms: 44 lbs ÷ 2.2 = 20 kgDose: 25 mg/kg × 20 kg = 500 mg The amount of medication available is 250 mg/5 mL. Therefore, to achieve a dose of 500 mg, the nurse will need to administer 10 mL of the medication.
Know more about Digoxin here:
https://brainly.com/question/12978309
#SPJ11
What are the half-reactions for a galvanic cell with Zn and Mg electrodes?
Answers
the half-reactions
cathode : Zn²⁺ (aq) + 2e⁻ ---> Zn (s)
anode : Mg (s) → Mg²⁺ (aq) + 2e−
a balanced cell reaction
Zn²⁺(aq) + Mg(s)→ Zn(s) + Mg²⁺ (aq)
Further explanationGiven
Zn and Mg electrodes
Required
The half-reactions for a galvanic cell
Solution
To determine the reaction of a voltaic cell, we must determine the metal that serves as the anode and the metal that serves as the cathode.
To determine this, we can either know from the standard potential value of the cell or use the voltaic series
1. voltaic series
Li-K-Ba-Ca-Na-Mg-Al-Mn- (H2O) -Zn-Cr-Fe-Cd-Co-Ni-Sn-Pb- (H) -Cu-Hg-Ag-Pt-Au
The more to the left, the metal is more reactive (easily release electrons) and the stronger reducing agent
So the metal on the left will easily undergo oxidation and function as anode
Since Mg is located to the left of Zn, then Mg functions as anode and Zn as a cathode
2. Standard potentials cell of Mg and Zn metals :
Mg2+ + 2e– → Mg E° = -2,35 V
Zn2+ + 2e– → Zn E° = -0,78 V
The anode has a smaller E°, then Mg is the anode and Zn is the cathode.
Answer:
Explanation:help
what is the symbol of the element in period 4 and group 2?
Answers
The element in period 4 and group 2 of the periodic table is calcium, with the chemical symbol "Ca". It is a highly reactive alkaline earth metal and plays vital roles in both geological and biological processes.
The element you are looking for, which is located in period 4 and group 2 of the periodic table, is calcium. Calcium has the chemical symbol "Ca" and is an alkaline earth metal. It is essential for various biological processes, including the formation of bones and teeth, blood clotting, and nerve impulse transmission. Calcium is also commonly found in various compounds such as calcium carbonate, which forms limestone and marble, and calcium sulfate, which is found in gypsum.
As a group 2 element, calcium has two valence electrons, which means it readily forms ionic compounds with a +2 charge. Due to its high reactivity, calcium is not typically found in its pure form in nature, but rather as part of different compounds. It is the fifth most abundant element in the Earth's crust and is also an essential nutrient for living organisms.
Learn more about periodic table here:-
https://brainly.com/question/31672126
#SPJ11
What happen when:
a. Magnesium Burns in oxygen.
b. Calcium carbonate is heated.
c. Hydrogen reacts with nitrogen at necessary condition.
d. potassium hydroxide reacts with nitric acid.
e. Carbon dioxide is treated with limewater.
Please help me to do this problem.
Answers
a. Magnesium Burns in oxygen.
--⟩it produces light bright enough to blind you temporarily.
b. Calcium carbonate is heated.
--⟩ form calcium oxide and evolve carbon dioxide gas.
c. Hydrogen reacts with nitrogen at necessary condition.
----⟩some of the hydrogen and nitrogen will react to form ammonia.
d. potassium hydroxide reacts with nitric acid.
---⟩When potassium reacts with nitric acid then, potassium displace hydrogen from its solution and becomes colorless liquid.
e. Carbon dioxide is treated with limewater.
---⟩to form a white precipitate (appears milky) of calcium carbonate, CaCO 3.
Hope it is helpful to you
500.0 mL of a gas is collected at 745 K. What will the volume be at standard temperature?
Answers
Answer:
183.22mL
Explanation:
Using Charles law equation, which is as follows:
V1/T1 = V2/T2
Where;
V1 = initial volume (mL)
T1 = initial temperature (K)
V2 = final volume (mL)
T2 = final temperature (K)
Note that standard temperature of a gas is 273K.
Hence, based on the provided information, V1 = 500.0mL, T1 = 745K, V2 = ?, T2 = 273K
Using the formula; V1/T1 = V2/T2
500/745 = V2/273
Cross multiply
V2 × 745 = 500 × 273
745V2 = 136500
V2 = 136500/745
V2 = 183.22
At standard temperature (273K), the new volume will be 183.22mL.
Answer:
183 ml
Explanation:
the diagram below shows the periodic table of the elements. Which pair of elements listed below has properties that are the most similar to each other?
explain why it’s the answer.

Answers
Calculate the mass of CuO which can react with 39,2 grams of orthophosphate acid.Please Help!!3CuO+ 2H3PO4 = Cu3(PO4)2 + 3H20
Answers
Answer
47.73 g CuO
Explanation
Given:
Chemical equation: 3CuO+ 2H3PO4 = Cu3(PO4)2 + 3H20
mass of orthophosphate acid (Cu3(PO4)2) = 39.2 g
Required: The mass of CuO
Solution:
\(\begin{gathered} 39.2g\text{ H}_3PO_4\text{ x }\frac{1\text{ mol H}_3PO_4}{97,994g\text{ H}_3PO_4}\text{ x }\frac{3\text{ moles CuO}}{2\text{ moles H}_3PO_4}\text{ x }\frac{79,545g\text{ CuO}}{1mole\text{ CuO}} \\ \\ =\text{ 47.73 g CuO} \end{gathered}\)Second method:
Step 1: Find the moles of H3PO4
n = m/M where m is the mass and M is the molar mass of H3PO4
n = 39.2g/97.994g.mol^-1
n = 0.400 mol
Step 2: Use the stoichiometry to find the moles of CuO
The molar ratio between CuO and H3PO4 is 3:2
Therefore the moles of CuO = 0.400 mol x (3/2) = 0.600 mol
Step 3: Find the mass of CuO, now that we have moles
m = n x M m is the mass, n is the moles and M is the molar mass
m = 0.600 mol x 79,545 g/mol
m = 47.73 g
Convert 8.7 g to mg
Answers
Hope I help
An insoluble solid that forms from a chemical reaction is called
Answers
Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The determining factors of the formation of a precipitate can vary.
describe it what is the scientefic method??

Answers
select the four most common types of molecules to which ncrnas bind
Answers
Non-coding RNAs (ncRNAs) are known to bind to a diverse array of molecules, facilitating various cellular processes. The four most common types of molecules to which ncRNAs bind are DNA, RNA, proteins, and small molecules. These interactions play crucial roles in gene regulation, chromatin remodeling, post-transcriptional modifications, and signaling pathways.
Non-coding RNAs (ncRNAs) are a group of RNA molecules that do not encode proteins but perform various regulatory functions in the cell. They can bind to different types of molecules, contributing to their functional roles. The first type of molecule to which ncRNAs commonly bind is DNA. Certain ncRNAs, such as long non-coding RNAs (lncRNAs), can bind to specific genomic regions, modulating gene expression and chromatin structure.
The second type of molecule is RNA itself. ncRNAs can interact with other RNA molecules through complementary base pairing, forming RNA-RNA complexes. For example, small interfering RNAs (siRNAs) and microRNAs (miRNAs) can bind to target messenger RNAs (mRNAs), resulting in their degradation or translational repression.
The third type of molecule that ncRNAs often bind to is proteins. ncRNAs can interact with proteins to form ribonucleoprotein complexes (RNPs). These interactions can influence protein localization, stability, and activity. Examples include the binding of small nuclear RNAs (snRNAs) to proteins to form spliceosomes, which are essential for mRNA processing.
Lastly, ncRNAs can also bind to small molecules, such as metabolites or signaling molecules. These interactions can regulate the activity of both the ncRNA and the small molecule, contributing to various cellular processes. For instance, certain riboswitches can bind to metabolites, leading to conformational changes in the RNA structure and modulating gene expression.
In conclusion, ncRNAs exhibit a remarkable versatility in their ability to bind to different types of molecules. Their interactions with DNA, RNA, proteins, and small molecules play pivotal roles in gene regulation, cellular signaling, and overall cellular homeostasis. Understanding these molecular interactions is crucial for unraveling the diverse functions of ncRNAs and their implications in health and disease.
To learn more about ncRNAs click here:
brainly.com/question/29725571
#SPJ11
What two questions remained about Wegener's ideas that kept scientists from accepting his theory
Answers
The two questions were:
What was the mechanism that caused the continents to move? How did the continents move without breaking up the Earth's crust? What are Wegener's ideas?Generally, Wegener's theory of continental drift, which proposed that the Earth's continents have moved over time and are still moving, was not immediately accepted by the scientific community when it was first proposed in the early 20th century. There were several reasons for this, but two of the main questions that remained about Wegener's ideas were:
What was the mechanism that caused the continents to move? Wegener proposed that the continents "drifted" on the Earth's surface, but he did not have a satisfactory explanation for the forces that would cause this movement.How did the continents move without breaking up the Earth's crust? Wegener's theory required the continents to plow through the rock of the Earth's crust as they moved, but this seemed unlikely given the solid nature of the crust.These questions and other issues with Wegener's theory kept most scientists from accepting it until the mid-20th century when the theory of plate tectonics was developed and provided a more complete and satisfactory explanation for the movement of the continents.
Read more about Wegener's ideas
https://brainly.com/question/14662728
#SPJ1
Describe the formation of oxygen molecule
Answers
Answer:
oxygen molecule has two oxygen atom . Each O atom share 2 electrons to form two covalent bonds out of which one is sigma bond and other is pi bond . sigma bond is formed by axial overlap 2p atomic orbitals of oxygen and pi bond is formed of lateral overlap of 2p atomic orbitals of oxygen .
What are the phases for the products of reaction I-2?CuSO4 5H2O (s) —> CuSO4 + 5H2OA. CuSO4 (aq) + 5H2O (l)B. CuSO4 (s) + 5H2O (l)C. CuSO4 (s) + 5H2O (aq)D. Ca+2H2O→ Ca(OH)2+H2
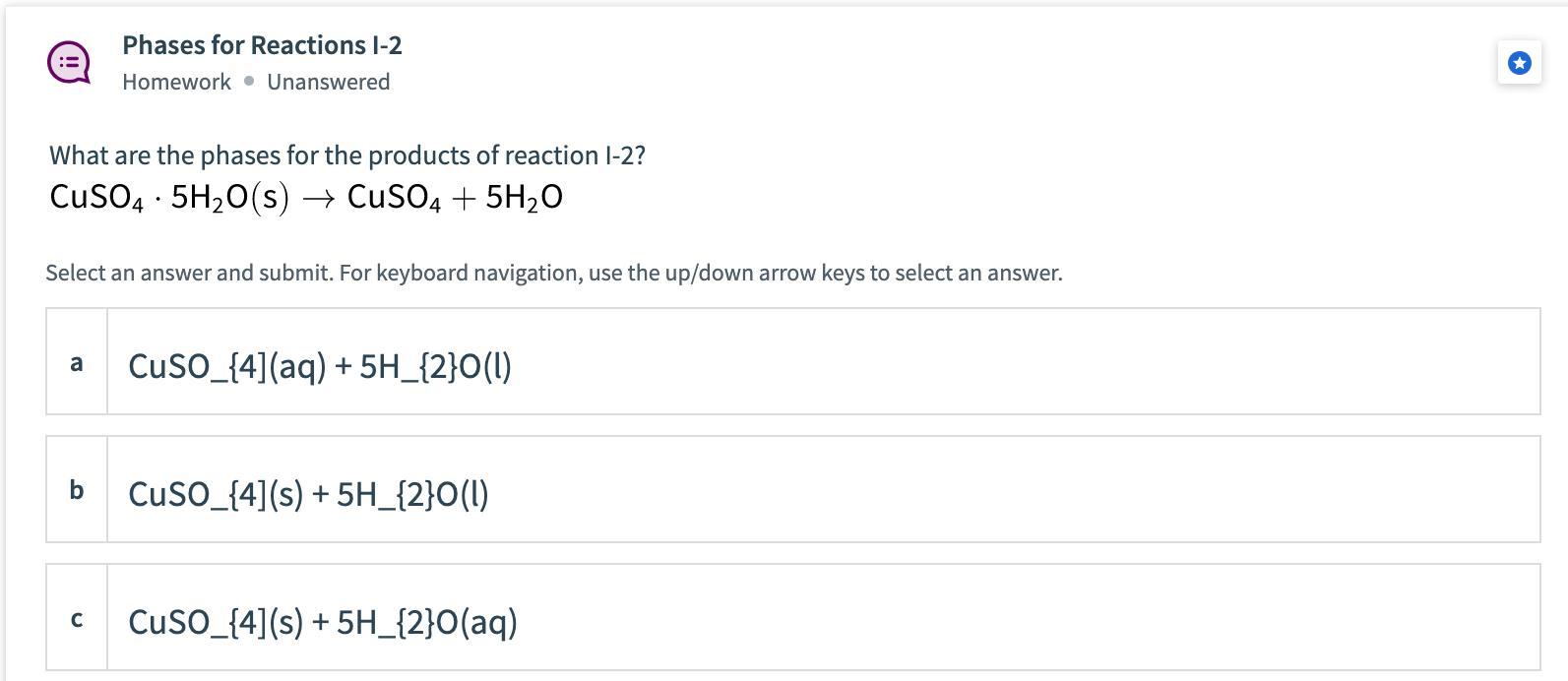
Answers
According to the given reaction, the phase of the salt, CuSO₄, is solid, it means that its subscript is (s).
The phase of water, H₂O, is liquid, it means that its subscripts is (l).
It means that the answer is B.
Why Ammonium Chloride formula is NH4Cl?
Answers
Ammonium chloride (NH4Cl), also known as 'sal ammoniac', is a salt of ammonia and hydrogen chloride. Its most common applications are as a nitrogen source in fertilisers and as an electrolyte in dry cells.
What are the uses of Ammonium chloride (NH4Cl)?
Ammonium chloride (NH4Cl) has several uses, including:
As a fertilizer, it is used to supply nitrogen to crops.In the food industry, it is used as a food additive and a source of salt.In the textile industry, it is used in printing and dyeing processes.It is also used as a fire extinguisher in laboratory settings.In electroplating, it is used as a source of chloride ion.In batteries, it acts as an electrolyte to allow the flow of electrical charges.In refrigeration systems, it is used as a refrigerant.As a curing agent, it is used in the production of silicone resins.In the pharmaceutical industry, it is used as an expectorant and cough suppressant.To learn more about Ammonium chloride (NH4Cl), visit: https://brainly.com/question/12969993
#SPJ4
Why is it preferable to use Am in smoke detector?
Low stable and highly reactive
High stability over long time and source need not be replaced
High penetration and high ionization
A good source of beta radiation and has a half-life of 432 years
Answers
Answer:
Ionization smoke detectors use americium as a source of alpha particles. Alpha particles from the americium source ionize air molecules. This makes some particles positively charged and some negatively charged. ... Because of this shielding, the smoke detector poses no radiation health risk when they are properly handled.
Explanation:
Hope this helped! :D
in water, 1 mol of li2co3 (aq) will dissociate into which ions?
Answers
1 mol of Li₂CO₃ (aq) will dissociate into two Li⁺ ions and one CO₃²⁻ ion. When Li₂CO₃ dissolves in water, the molecules of the salt separate and the Li⁺ and CO₃²⁻ ions are formed.
The two Li⁺ ions become surrounded by water molecules, forming hydrated ions. The CO₃²⁻ ions are not hydrated, so they remain as free ions in solution. The ionic bonding between the Li⁺ and CO₃²⁻ ions is broken due to the strong polarizing power of the water molecules.
The Li⁺ and CO₃²⁻ ions then move freely in the solution due to the electrostatic repulsive forces between them. These ions also interact with the water molecules and the other ions present in the solution.
The Li⁺ and CO₃²⁻ ions are then further dispersed throughout the solution, forming a homogeneous solution of the salt.
To know more about polarizing power click on below link:
https://brainly.com/question/15189464#
#SPJ11
Which of the following are properties of metalloids?
A. Semi-conductors
B. All of these
C. React like metals sometimes
D. React like non-metals sometimes
Answers
Answer:
all of these are properties of metalloids
Answer:
The answer is B. All of these
Explanation:
Can you mark me the brainliest?
Without any calculations, determine which solution in each pair is more basic.
Part A
a.0.100 M in KClO
b. 0.100 M in NaF
Part B
a. 0.0100 M in NaBrO
b. 0.0100 M in NaBr
Part C
a. 0.0100 M in HNO_2
b. 0.0100 M in KOH
Part D
a. 0.0100 M in NH_4Cl
b. 0.0100 M in HCN
Answers
In each pair, the solution that contains the weaker conjugate acid is more basic. Without any calculations, we can determine which solution is more basic by identifying the stronger conjugate acid in each pair.
In Part A, KClO is a stronger acid than NaF, so the solution in (b) is more basic in Part B, NaBrO is a stronger acid than NaBr, so the solution in (b) is more basic in Part C, HNO2 is a weaker acid than KOH, so the solution in (b) is more basic in Part D, NH4Cl is a weaker acid than HCN, so the solution in (a) is more basic.
It is important to note that while we did not perform any calculations, this method only works for comparing solutions with the same concentration. If the concentrations were different, we would need to perform calculations to determine which solution is more basic. A
To know more about conjugate acid visit:
https://brainly.com/question/31229565
#SPJ11
T/F: prochirality center desrcibes an sp3 hybridized atom that can become a chirality center by changing one of its attached groups
Answers
False. A prochiral center does not describe an sp_3 hybridized atom that can become a chirality center by changing one of its attached groups.
A prochiral center is an atom that possesses chirality, meaning it can become a chirality center by changing its stereochemistry. However, the statement in question is incorrect because a prochiral center does not require changing one of its attached groups to become a chirality center.
In contrast, a prochiral center is a type of stereocenter that exhibits chirality due to the presence of two different groups attached to it. It becomes a chirality center when one of the groups is replaced by another group, resulting in the formation of two distinct stereoisomers.
An example of a prochiral center is a carbon atom with three different groups attached to it. Upon substitution of one of the groups, the prochiral center becomes a chirality center, giving rise to enantiomers.
Therefore, the statement that a prochiral center can become a chirality center by changing one of its attached groups is false.
Learn more about prochiral from this link:
https://brainly.com/question/31486480
#SPJ11
Can someone pls help me with this question?:
Rutherford could not find all the mass of the nucleus during his first observations. Describe the discovery that helped explain this phenomenon?
I appreciate your help!
Answers
Answer:
James Chadwick
Explanation:
In his first experiment, Rutherford was unable to predict the total mass of nucleus. He noticed that if only electrons and protons are present in the nucleus then by adding their masses, it falls short of the net mass of the atom. so he concluded that there must be another particle and that Particle was later discovered by James Chadwick in 1932.
What happens to climate and weather over a summer?
Answers
Answer:
summers get hotter, and it can get more sticky or humid sometimes.
Explanation:
think of the seasons and the tempatures.
How is potassium sulphate formula formed?
Answers
Potassium sulphate, also known as sulphate of potash or arcanite, is a chemical compound made up of potassium, sulfur, and oxygen. The chemical formula for potassium sulphate is K2SO4.
The formation of potassium sulphate starts with potassium chloride (KCl), which is a naturally occurring mineral that is commonly found in evaporite deposits, such as those found in ancient lakes and seas. Potassium chloride is first heated to a high temperature, typically around 816-900 °C, in the presence of sulfuric acid (H2SO4) in order to form potassium sulphate. The chemical reaction that takes place can be represented by the following equation:
KCl + H2SO4 → K2SO4 + HCl
In this equation, potassium chloride (KCl) reacts with sulfuric acid (H2SO4) to form potassium sulphate (K2SO4) and hydrogen chloride (HCl) as the by-product. This process is known as the "chamber process" and it is the most commonly used method for the industrial production of potassium sulphate.
Potassium sulphate is an important chemical compound used in many industrial and agricultural applications. It is a key ingredient in fertilizers, and is also used in the manufacturing of glass, ceramics, and soap. Additionally, it is also used as a food additive, and as a component of some medications.
TO know more about potassium sulphate here:
https://brainly.com/question/30111542#
#SPJ11