a galvanic cell cannot generate electricity forever. list two chemical reactions you can think of for why a galvanic cell may go dead.
Answers
A galvanic cell is a type of electrochemical cell that produces electrical energy from spontaneous redox(both oxidisation and reduction) reactions taking place in the cell. It has two half-cells connected by a salt bridge, and each half-cell contains an electrode and an electrolyte.
A galvanic cell cannot generate electricity forever because the chemical reactions occurring inside the cell eventually reach equilibrium or the reactants get depleted. Two chemical reactions that contribute to a galvanic cell going dead are:
1. Oxidation reaction: In this reaction, a substance loses electrons and gets oxidized. This occurs at the anode of the galvanic cell. Over time, the reactants participating in the oxidation reaction will be depleted, causing the galvanic cell to go dead.
2. Reduction reaction: This reaction occurs at the cathode of the galvanic cell, where a substance gains electrons and gets reduced. As with the oxidation reaction, the reactants involved in the reduction reaction will also get depleted over time, eventually causing the galvanic cell to stop producing electricity.
In summary, a galvanic cell goes dead due to the depletion of reactants in the oxidation and reduction reactions occurring within the cell.
Learn more about galvanic cell here,https://brainly.com/question/15096829
#SPJ11
Related Questions
why do molecules and atoms speed up when the amount of heat increases
Answers
Heat is the energy that a thing possesses as a result of the movement of its atoms and molecules, which are always bouncing off of one another and other objects. An object's atoms and molecules move more quickly when we add energy to it, which increases the object's energy of motion or heat.
The gas will travel more quickly in the container if the temperature is raised. This occurs as a result of the particles' increased kinetic energy caused by heating. There will be more collisions between particles if they are traveling faster.
A substance's atoms and molecules move more quickly when it is heated. Whether the substance is a solid, liquid, or gas, this occurs.
To know more about heat energy, visit;
https://brainly.com/question/13411214
#SPJ6
Liquids with many free hydroxide ions (OH-) are called _________.
Answers
Answer:
hypothesis testing center of the year with 32 days
A cell placed in a solution of unknown composition soon swelled up and burst, so the solution must have been __________ to the cell when it was originally placed in the solution.
Answers
The correct option is Hypotonic. A cell that swelled up and burst indicates that the cell was initially placed in a hypotonic solution. Hypotonic solution is a solution in which the concentration of solutes is lower than that of the cell.
When a cell is placed in a hypotonic solution, water moves into the cell due to the concentration gradient, which causes the cell to swell up. The cell wall protects the cell from bursting, but animal cells do not have a cell wall to protect them, which causes them to burst. In a hypertonic solution, the concentration of solutes is higher than that of the cell.
When a cell is placed in a hypertonic solution, water moves out of the cell causing the cell to shrink. In an isotonic solution, the concentration of solutes is the same as that of the cell. When a cell is placed in an isotonic solution, there is no net movement of water in or out of the cell. Therefore, the solution must have been hypotonic to the cell when it was originally placed in the solution.
To know more about Hypotonic visit :
https://brainly.com/question/28020628
#SPJ11
A sample of gas has a volume of 215 cm3 at 23.5 °C and 3 atm. What will the volume of the gas be at STP
Answers
Answer:
165.3 cm^3
Explanation: hope this is correct!!
P1 * V1 / T1 = P2 * V2 / T2
P1 = 84.6 kPa
V1 = 215 cm³
T1 = 23.5°C = 23.5 + 273 K = 296.5 K
At STP:
P2 = 101.3 kPa
V2 = ?
T2 = 273 K
The synthesis of maleic acid anhydride (CH₂O₂) can be accomplished
by reacting benzene (CH) and oxygen gas in the following chemical
reaction:
2 CH (1) +9 O₂(g) → 2 C₂H₂O₂(s) + 4 CO₂(g) + 4H₂O(g)
What is the mass in grams of oxygen gas that is required to produce
52.1 grams of maleic acid anhydride?
Answers
The mass of oxygen is 76.32 g
How does stoichiometry affect a reaction?Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction. It refers to the mole ratios of the reactants and products involved in a reaction.
We know that;
Number of moles of maleic acid anhydride = 52.1 grams /98 g/mol
= 0.53 moles
If 9 moles of oxygen produces 2 moles of maleic acid anhydride
x moles of oxygen will produce 0.53 moles of maleic acid anhydride
x = 2.385 moles
Mass of oxygen = 2.385 moles * 32 g/mol
= 76.32 g
Learn more about mass of oxygen:https://brainly.com/question/26789700
#SPJ1
how could two objects change in order for there to be a stronger electric force between them?
Answers
Answer:
Increasing the separation distance between objects decreases the force of attraction or repulsion between the objects.
What is the rate constant of a first-order reaction that takes 354 seconds for the reactant concentration to drop to half of its initial value?
Answers
The rate constant of a first-order reaction can be calculated using the formula k = ln(2) / t, where k is the rate constant and t is the time it takes for the reactant concentration to drop to half of its initial value.
In this case, the time given is 354 seconds. Using the formula, we can calculate the rate constant:
k = ln(2) / 354
k ≈ 0.00196 s^-1
The rate constant of a first-order reaction represents the speed at which the reaction occurs. It is specific to each reaction and is independent of the initial concentration of the reactant. In this case, the rate constant is approximately 0.00196 s^-1.
The rate constant of a first-order reaction is an important parameter in chemical kinetics. It determines the rate at which the reaction proceeds.
In a first-order reaction, the rate of reaction is directly proportional to the concentration of the reactant. As the reactant concentration decreases, the rate of reaction decreases. The rate constant is calculated by using the natural logarithm of 2 divided by the time it takes for the reactant concentration to halve. In this case, the given time is 354 seconds. Plugging this value into the formula, the rate constant is approximately 0.00196 s^-1. This means that the reaction proceeds at a rate of 0.00196 units per second. The rate constant is a characteristic of the specific reaction and can be used to determine the reaction kinetics and predict the reaction's behavior under different conditions.
To know more about reaction visit:
https://brainly.com/question/16737295
#SPJ11
how many millimoles of bromine is in 0.5 ml of 1 m solution in ch2cl2
Answers
There are 0.5 millimoles of bromine in 0.5 ml of a 1 m solution in CH2Cl2.
To find out how many millimoles of bromine are in 0.5 ml of a 1 m solution in CH2Cl2, we need to use the formula:
millimoles = moles x 1000
First, we need to find the moles of bromine in the solution. We know that the solution is 1 molar, which means that it contains 1 mole of bromine per liter of solution. Since we only have 0.5 ml of the solution, we need to convert this to liters:
0.5 ml = 0.0005 L
Now we can calculate the number of moles of bromine in the solution:
moles = concentration x volume
moles = 1 mol/L x 0.0005 L
moles = 0.0005 mol
Finally, we can convert this to millimoles using the formula above:
millimoles = moles x 1000
millimoles = 0.0005 mol x 1000
millimoles = 0.5 millimoles
Therefore, there are 0.5 millimoles of bromine in 0.5 ml of a 1 m solution in CH2Cl2.
Learn more about bromine here:
https://brainly.com/question/29028678
#SPJ11
29. A branched chain amino acid is a. Cys b. Leu c. Glu d. Lys 30. An aa often involved in Redox reactions is a. Cvs b. Leu c. Glu d. Lys 31. The minimum number of electrons that FAD can carry is a. 1 b. 2 c. 3 d. 4 32. NAD carries a. protons b. electrons c. hydride 33. The aa with the highest tendency to make a-helices is a. Gly b. Pro c. Ala 34. A common residue in type I b-turns is a. a. Gly b. Pro c. Ala www d. hydrogen atoms d. Leu www. d. Leu
Answers
30. A branched-chain amino acid is (b) Leu (Leucine). Branched-chain amino acids have a non-linear or branched side chain structure. Leucine is one of the three branched-chain amino acids commonly found in proteins.
31. An amino acid often involved in redox reactions is (d) Lys (Lysine). Lysine contains a side chain with an amino group and a positively charged amino group, which can participate in electron transfer during redox reactions.
32. The minimum number of electrons that FAD (Flavin adenine dinucleotide) can carry is (b) 2. FAD is a redox-active coenzyme involved in various biological processes, including carrying and transferring electrons.
33. The amino acid with the highest tendency to form α-helices is (c) Ala (Alanine). Alanine is a small, non-polar amino acid that readily fits into the α-helix structure due to its conformational flexibility and favorable interactions with neighboring amino acids.
34. A common residue in type I β-turns is (b) Pro (Proline). Proline is often found in the second position of type I β-turns due to its unique cyclic structure, which helps induce the sharp turn required for this secondary structure motif.
In conclusion, the answers to the given questions are:
30. (b) Leu
31. (d) Lys
32. (b) 2
33. (c) Ala
34. (b) Pro
These amino acids and their characteristics play important roles in protein structure, function, and various biochemical processes in living organisms.
To know more about branched visit:
https://brainly.com/question/5023567
#SPJ11
Which of the following is not a characteristic of a solution?What is the concentration of a solution?
Answers
Answer: B. it will scatter a beam of light
Explanation: hope it is helpful........
(a) what is meant by diffusion? give one example of diffusion in gases.
(b) why do gase diffuse very fast?
(d) Name two gases of air which dissolve in water by diffusion. what is the importance of this process in nature?
please answer it fast
Answers
b) Gaseous particles tend to undergo diffusion because they have kinetic energy.
Answer:
The gases which dissolve in water by diffusion are carbon dioxide and oxygen.
Explanation:
HAVE A GOOD DAY!
Electrical Bonding and Kinetic Energy Quick Check
2. Which statement describes the effect of adding more energy to a system, assuming a phase change does not occur?(1 point)
A. The particles within the system will have greater motion, and the temperature will decrease.
B. The particles within the system will have less motion, and the temperature will increase.
C. The particles within the system will have less motion, and the temperature will decrease.
D. The particles within the system will have greater motion, and the temperature will increase
3. Which statement correctly describes how attractions that hold particles break?(1 point)
A. Attractions occur due to gravitational forces. When particles have low enough energy, these forces can no longer keep particles together.
B. Attractions occur due to electrostatic forces. When particles have low enough energy, these forces can no longer keep particles together.
C. Attractions occur due to electrostatic forces. When particles move fast enough, these forces can no longer keep particles together.
D. Attractions occur due to gravitational forces. When particles move fast enough, these forces can no longer keep particles together.
4. Which statement explains why a rubber band analogy is not a perfect comparison for bonds in a substance when considering phase changes?(1 point)
A. For a phase change from solid to liquid, the bonds break completely and particles can move independently of each other.
B. For a phase change from solid to liquid, the bonds do not break completely and particles can still slide past each other.
C. For a phase change from liquid to gas, the bonds do not break completely and particles can still slide past each other.
D. For a phase change from liquid to gas, the bonds break completely and particles can move independently of each other.
5. The boiling point of benzene is 80ºC. Which pair of samples will have the same average kinetic energy as benzene molecules?(1 point)
A. a sample of liquid benzene at 70ºC and a sample of gaseous benzene at 90ºC
B. two samples of liquid benzene, one at 70ºC and the other at 80ºC
C. two samples of gaseous benzene, one at 80ºC and the other at 90ºC
D. a sample of liquid benzene at 80ºC and a sample of gaseous benzene at 80ºC
Answers
When particles move fast enough, these forces can no longer keep particles together.
Recall that the particles of a substance are in constant random motion. Hence, when more energy is added to the system, the particles within the system will have greater motion, and the temperature will increase.
The forces that keep molecules together in a particular state of matter are electrostatic forces. When particles move fast enough, these forces can no longer keep particles together.
It is common to use the analogy of an elastic material to represent the bonding between atoms in molecules. However, this analogy is not apt in a phase change because, for a phase change from liquid to gas, the bonds break completely and particles can move independently of each other.
We know that the average kinetic energy of the molecules of a substance depends on the temperature of the body. Therefore, a sample of liquid benzene at 80ºC and a sample of gaseous benzene at 80ºC will have the same average kinetic energy as benzene molecules.
Learn more: https://brainly.com/question/6284546
which is the best practice recommended in the safety video to mix an acid or a base with a solvent?group of answer choicesyou pour them at the same timeadd acid or base to the solventadd solvent to the acid or basethe order is irrelevant
Answers
The best practice recommended in the safety video for mixing an acid or a base with a solvent is to add the acid or base to the solvent.
In the safety video, it is emphasized that adding acid or base to the solvent is the safest method. This is because if the solvent is added to the acid or base, there is a higher chance of splashing or overflowing, which could lead to a dangerous situation. It is important to also stir the mixture slowly and carefully while adding the acid or base to the solvent to ensure it is fully mixed before using it.
Additionally, wearing appropriate personal protective equipment such as gloves and safety goggles is crucial when handling chemicals. By following these safety guidelines, the risk of accidents or injury can be minimized while mixing acid or base with a solvent.
To learn more about solvent click brainly.com/question/12237541
#SPJ11
A radiation source of 1000 watts is located at a point in space. What is the intensity of radiation at a distance of 10 meters form the source
Answers
The intensity of radiation from a source follows an inverse square law, which means that as the distance from the source increases, the intensity decreases.
Given:
Power of the radiation source = 1000 watts
Distance from the source = 10 meters
The intensity (I) of radiation is defined as the power (P) per unit area (A):
Intensity = Power / Area
Since we are not given the specific area, we need to make an assumption. Let's assume that the radiation is spreading out equally in all directions, forming a spherical wavefront.
The surface area of a sphere is given by the formula:
Area = 4πr^2
Where r is the distance from the source.
Plugging in the values:
Area = 4π(10)^2 = 400π square meters
Now we can calculate the intensity:
Intensity = Power / Area
Intensity = 1000 watts / 400π square meters
To round the answer to three significant figures, we can use 3.14 as an approximation for π.
Intensity ≈ 1000 watts / (400 * 3.14) square meters
Intensity ≈ 0.795 watts per square meter
Therefore, at a distance of 10 meters from the source, the intensity of radiation is approximately 0.795 watts per square meter.
To learn more about radiation click here:brainly.com/question/31106159
#SPJ11
draw one possible dipeptide that is formed between alanine and leucine, as the zwitterion.
Answers
To form a dipeptide between Alanine and Leucine, we have to join the carboxyl group (COOH) of Alanine with the amino group (NH₂) of Leucine via a peptide bond. The resulting molecule will have a zwitterionic form. The zwitterionic form of the dipeptide will have both a positive and a negative charge.
A dipeptide is a molecule made up of two amino acid residues joined together via a peptide bond. A peptide bond is a bond between the amino group (NH₂) of one amino acid and the carboxyl group (COOH) of another amino acid. Amino acids are the building blocks of proteins. Alanine and Leucine are two of the twenty common amino acids found in nature.
A zwitterion is a molecule that has a positive charge on one part of the molecule and a negative charge on another part of the molecule. Zwitterions are electrically neutral overall. They are formed when a molecule that has both acidic and basic functional groups is dissolved in a solvent. The acidic and basic groups react with each other to form a neutral molecule that has both positive and negative charges. The zwitterionic form of an amino acid is the form that is found in proteins.
The chemical formula for Alanine is C₃H₇NO₂, and the chemical formula for Leucine is C₆H₁₃NO₂. To form a dipeptide between Alanine and Leucine, we have to join the carboxyl group (COOH) of Alanine with the amino group (NH₂) of Leucine via a peptide bond. The resulting molecule will have a zwitterionic form. The zwitterionic form of the dipeptide will have both a positive and a negative charge.
To know more about dipeptide, refer
https://brainly.com/question/31524411
#SPJ11
Estimate the average distance between molecules in air at 0.0^{\circ} {C} and 5.00 atm.
Answers
The estimated average distance between molecules in air at 0.0°C and 5.00 atm is approximately 11.34 nanometer
To estimate the average distance between molecules in air at 0.0°C and 5.00 atm, we can use the ideal gas law and some simplifying assumptions.
The ideal gas law relates pressure (P), volume (V), number of moles (n), and temperature (T) of a gas:
PV = nRT
Where R is the ideal gas constant. Rearranging the equation, we get:
V = (nRT) / P
Assuming air behaves as an ideal gas under these conditions, we can use the molar volume of an ideal gas at standard temperature and pressure (STP) to estimate the volume per mole of gas. At STP, the molar volume is approximately 22.4 liters/mole.
Now, let's calculate the average distance between molecules. We know that the average distance (d) between molecules is inversely proportional to the molar concentration (C), which is given by:
C = n / V
Rearranging the equation, we get:
d = V / n
Substituting the expression for V, we have:
d = (nRT) / (nP) = RT / P
Using the ideal gas constant R = 0.0821 L·atm/(K·mol) and the given values of temperature T = 0.0°C = 273.15 K and pressure P = 5.00 atm, we can calculate the average distance:
d = (0.0821 L·atm/(K·mol)) * (273.15 K) / (5.00 atm)
d ≈ 11.34 nm (nanometers)
Therefore, the estimated average distance between molecules in air at 0.0°C and 5.00 atm is approximately 11.34 nanometer
Learn more about Average Distance at
brainly.com/question/3841552
#SPJ4
Use the tabulated electrode potentials to calculate KK for the oxidation of zinc by H+H+ (at 25 ∘C∘C):Zn(s)+2H+(aq)→Zn2+(aq)+H2(g)
Answers
The KK for the oxidation of zinc by hydrogen ions at 25 °C is -1.23 V.
The standard reduction potentials for the half-reactions involved in the oxidation of zinc by hydrogen ions can be used to calculate the Nernst constant (KK) for the reaction at a given temperature using the following equation:
KK = \(e^{(-E/RT)} / [1 + e^{(-E/RT)]\)
The standard reduction potential for the half-reaction \(Zn(s)+2H+(aq) == Zn_2+(aq)+H_2(g) is -0.76 V.\)
The standard reduction potential for the half-reaction :\(Zn(s)+2H+(aq) == Zn_2+(aq)+H_2(g) is -0.76 V.\)
The standard reduction potential for the half-reaction H(g)+4e-→2H*(-2) is -2.44 V.
Using the tabulated electrode potentials, we can find the standard reduction potential for the half-reaction:\(Zn(s)+2H+(aq) == Zn_2+(aq)+H_2(g) is -0.76 V.\)
Standard reduction potential (E°) = -0.76 V
Standard reduction potential (E°) = -0.76 V
The standard reduction potential for the half-reaction:\(Zn(s)+2H+(aq) == Zn_2+(aq)+H_2(g) is -0.76 V.\)
Standard reduction potential (E°) = -2.44 V
Using the equation for KK, we can calculate the KK for the oxidation of zinc by hydrogen ions at 25 °C:
KK = \(e^{(-E/RT)} / [1 + e^{(-E/RT)]\)
\(KK = e^{(-(-0.76 V)/(298 K * 1 atm)) }/ [1 + e^{(-(-0.76 V)/(298 K * 1 atm))]\\KK = e^{(-0.76 V)}/(1.105 + e^{(-0.76 V))\)
KK = -1.23 V
Therefore, the KK for the oxidation of zinc by hydrogen ions at 25 °C is -1.23 V.
Learn more about oxidation visit: brainly.com/question/13182308
#SPJ4
What is ionic bond and explain it
Answers
Answer:
An ionic bond is a chemical bonding involving the attraction between oppositely charged ions
Explanation:
On the periodic table, elements from group 1 and 7 are attracted to each other and when they bond, it's called ionic bonding. This is because of their valence electrons and ions.
Answer:
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding along with covalent bonding.
Iodine adds to the double bonds in fatty acids (one iodine molecule per double bond). How many double bonds are in a molecule of arachidonic acid (Molar mass
Answers
Answer:
To test if a lipid is saturated or unsaturated iodine is added. If the iodine changes from brown to clear the lipid is unsaturated. If the iodine does not change colors the lipid is saturated. To test for the degree of lipid saturation iodine is added to the unsaturated lipid.
Explanation:
henol, (c6h5oh) , has a ka of 1.3×10−10 . part a write out the ka reaction for phenol. express your answer as a chemical equation including phases.
Answers
The Ka reaction for phenol
C6H5OH (aq) + H2O (l) ⇌ C6H5O- (aq) + H3O+ (aq)
Phenol (C6H5OH) is a weak acid that partially dissociates in aqueous solution to form phenoxide ion (C6H5O-) and hydronium ion (H3O+). The dissociation of phenol can be represented by the following equation:
C6H5OH (aq) + H2O (l) ⇌ C6H5O- (aq) + H3O+ (aq)
where (aq) denotes aqueous phase and (l) denotes liquid phase. The equilibrium constant for this reaction is the acid dissociation constant (Ka) of phenol and is equal to 1.3×10−10. This value indicates that phenol is a weak acid because it only partially dissociates in aqueous solution.
For more questions like Reaction click the link below:
https://brainly.com/question/28984750
#SPJ11
Which subatomic particles have approximately the same mass?
protons and electrons
neutrons and electrons
protons and neutrons
electrons and atoms
Answers
Explanation:
protons and neutrons have approximately the same size, the electron is 1847th, (I think), the size of a proton
The subatomic particles which are having approximately the same mass are neutrons and protons. Thus, option c is correct.
What are subatomic particles?An atom is made of subatomic particles namely neutrons, protons and electrons. The neutrons and protons are located inside the nucleus whereas, the electrons are revolving around the nucleus through circular paths definite energy.
The electrons are having negative charges and protons are of positive charge. For a neutral atom the number of electrons and protons are equal. Electrons have negligible mass.
The mass of an atom is mainly contributed by the nucleus. The mass of neutrons and protons are have approximately same mass. Therefore, option c is correct.
To find more on atomic mass, refer here:
https://brainly.com/question/29117302
# SPJ2
Having a control group is important when doing an investigation why?
Answers
Answer:to help understand the stuff more
Explanation:
Answer :
Dependent variable depends on the independent variable. ... In a control group in an experimental investigation the independent variable is omitted. Thus the goal is to test if the dependent variable shows any change in the absence of the independent variable.
Choose the correct formula for each acid.
Carbonic acid
-HC
-H2CO3
-HCO
Answers
Answer:
H₂CO₃
Explanation:
Water + Carbon di oxide
H20+ CO2
= H2CO3
What will the ph at the neutralization point of 0. 00812 m ba(oh)2 be when titrated with hcl?
Answers
The pH at the neutralization point of 0.00812 M Ba(OH)2 when titrated with HCl will be 7.
At the neutralization point of a titration, the moles of acid and base are stoichiometrically balanced, resulting in the formation of a neutral salt and water. In this case, 0.00812 M Ba(OH)₂ is the base being titrated with HCl, which is an acid.
Barium hydroxide (Ba(OH)₂) is a strong base, while hydrochloric acid (HCl) is a strong acid. When the base reacts with the acid, the hydroxide ions (OH-) from Ba(OH)₂ combine with the hydrogen ions (H+) from HCl to form water (H₂O). The resulting salt is barium chloride (BaCl₂).
At the neutralization point, all the hydroxide ions have reacted with the hydrogen ions, resulting in the complete formation of water. Since water is neutral, the pH at the neutralization point is 7, which is considered neutral on the pH scale.
In summary, the pH at the neutralization point of 0.00812 M Ba(OH)₂ when titrated with HCl is 7, indicating a neutral solution.
Learn more about : pH.
brainly.com/question/2288405
#SPJ11
Two solid chemical compounds are mixed together in a beaker. After one minute, ice crystals are observed on the outside of the beaker. What is the best description for the energy change occurring with the reaction inside the beaker?
Group of answer choices
exothermic because heat is being released to the surroundings
endothermic because heat is being released to the surroundings
exothermic because heat is being absorbed from the surroundings
endothermic because heat is being absorbed from the surroundings
Answers
The description that fits the reaction that was observed is endothermic because heat is being absorbed from the surroundings. Option D
What more should you know about endothermic reaction?Endothermic reaction stores energy. In the reaction that has occurred, heat energy was absorbed from the enviroment which makes the beaker to become cold.
Assuming it was an exothermic reaction, heat energy would have been released to the surrounding of the beaker. the beaker would have felt warm or hot to the touch,
Find more exercises on endothermic reaction;
https://brainly.com/question/10373907
#SPJ1
would you expect the ph of .25 m acetic acid to be higher or lower than the ph of .25 m hydrochloric acid solution
Answers
The pH of a .25 M acetic acid solution would be higher than the pH of a .25 M hydrochloric acid solution. This is because acetic acid is a weak acid, meaning it only partially dissociates in water, while hydrochloric acid is a strong acid, meaning it completely dissociates in water. The pH of a weak acid solution is higher than the pH of a strong acid solution of the same concentration.
Based on the terms you provided, I'll compare the pH of 0.25 M acetic acid and 0.25 M hydrochloric acid solutions.
Acetic acid is a weak acid, which means it doesn't completely dissociate in water, whereas hydrochloric acid is a strong acid, meaning it fully dissociates in water. When an acid dissociates, it releases hydrogen ions (H+) into the solution, which determines the pH.
Here's a step-by-step comparison:
1. A 0.25 M acetic acid solution will only partially dissociate, releasing fewer H+ ions into the solution.
2. A 0.25 M hydrochloric acid solution will completely dissociate, releasing more H+ ions into the solution.
Since the pH scale is logarithmic and inversely related to the concentration of H+ ions, a solution with fewer H+ ions will have a higher pH (less acidic) than a solution with more H+ ions.
Therefore, you would expect the pH of a 0.25 M acetic acid solution to be higher (less acidic) than the pH of a 0.25 M hydrochloric acid solution.
For more information on hydrochloric acid visit:
brainly.com/question/15231576
#SPJ11
how many ml of a 0.121 m aqueous solution of iron(iii) acetate, , must be taken to obtain 6.79 grams of the salt?
Answers
242 ml of a 0.121 m aqueous solution of iron(iii) acetate, must be taken to obtain 6.79 grams of the salt
Determining the quantity of Iron acetate solution:
To determine how many ml of a 0.121 M aqueous solution of iron(III) acetate are needed to obtain 6.79 grams of the salt, follow these steps:
1. Calculate the molar mass of iron(III) acetate: Fe(C2H3O2)3
Fe: 55.85 g/mol
C2H3O2: (2 * 12.01) + (3 * 1.01) + (2 * 16.00) = 59.05 g/mol
Molar mass of Fe(C2H3O2)3: 55.85 + (3 * 59.05) = 232.00 g/mol
2. Calculate the number of moles of iron(III) acetate in 6.79 grams:
moles = mass / molar mass = 6.79 g / 232.00 g/mol ≈ 0.0293 mol
3. Calculate the volume of the 0.121 M aqueous solution needed:
Volume = moles/molarity = 0.0293 mol / 0.121 M ≈ 0.242 ml
Therefore, you need approximately 242 ml of a 0.121 M aqueous solution of iron(III) acetate to obtain 6.79 grams of the salt.
To know more about the aqueous solution, visit: https://brainly.com/question/26856926
#SPJ11
What’s the answer?????
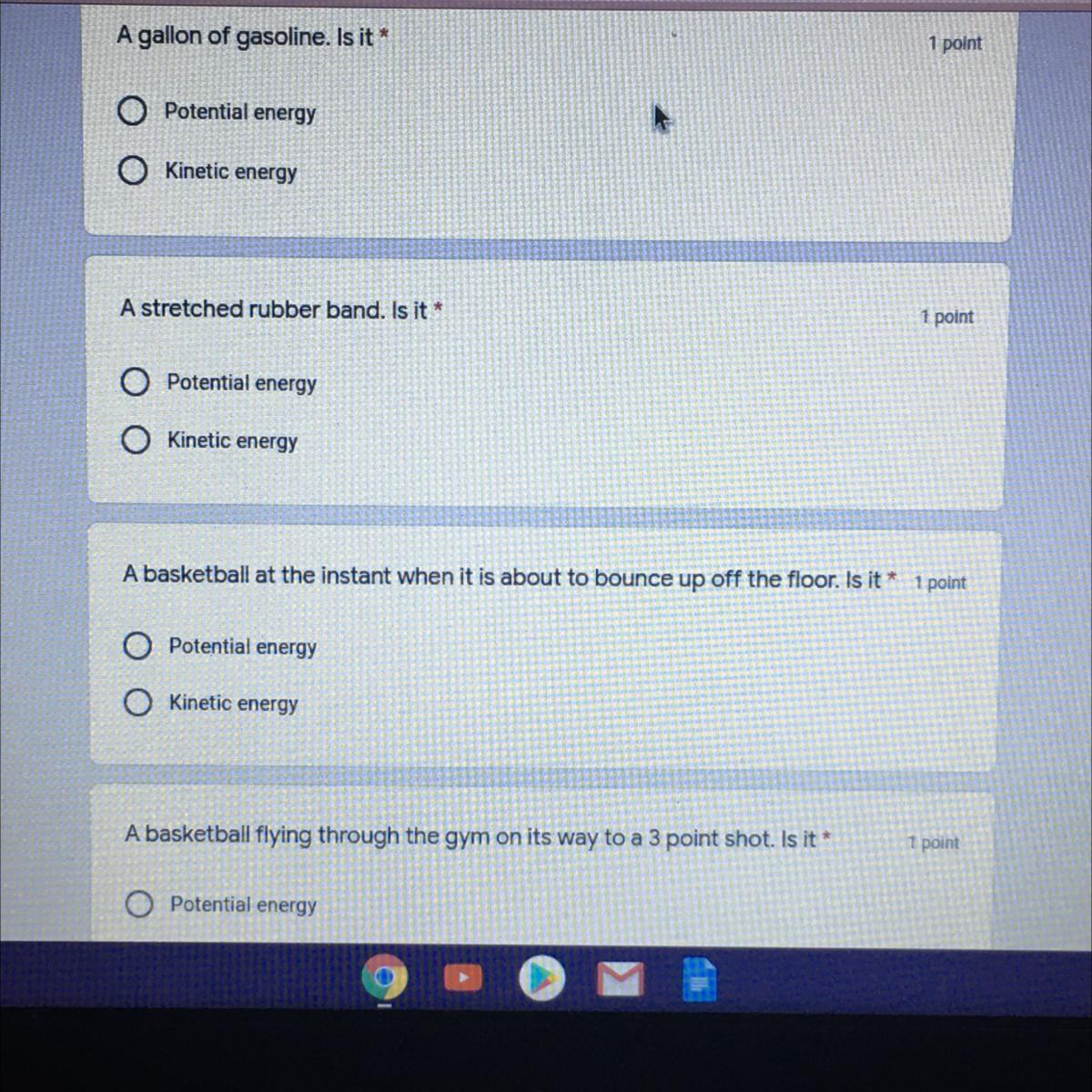
Answers
potential energy
kinetic energy
kinetic energy
idk last sorry =(
consider the reaction below: a series of experiments using a solution of was heated at different temperatures. after some time, the data below were obtained. answer the following questions: use what is the activation energy ( ) for this reaction?
Answers
The activation energy for this reaction is 64.5 kJ/mol.
To determine the activation energy (Ea) for a reaction, we need to use the Arrhenius equation. This equation relates the rate constant (k) to the temperature (T) and the activation energy of the reaction. The equation is as follows:
k = Ae^(-Ea/RT)
where A is the pre-exponential factor, R is the gas constant, and T is the temperature in Kelvin.
We have been given the data for a series of experiments where a solution was heated at different temperatures. The data should include the rate of reaction (k) at each temperature.
To calculate the activation energy, we need to use two sets of data: the rate constant (k) and the temperature (T) for two experiments. We can then substitute these values into the Arrhenius equation and solve for Ea.
Let's say we have two sets of data:
k1 = 0.1 s^-1, T1 = 300 K
k2 = 0.4 s^-1, T2 = 350 K
Substituting these values into the Arrhenius equation, we get:
ln(k1/k2) = (Ea/R)(1/T2 - 1/T1)
Solving for Ea, we get:
Ea = -R ln(k1/k2)/(1/T2 - 1/T1)
Plugging in the values, we get:
Ea = -8.31 J/mol K ln(0.1/0.4)/(1/350 - 1/300)
Ea = 64.5 kJ/mol
Therefore, 64.5 kJ/mol is the activation energy.
Know more about activation energy here:
https://brainly.com/question/28384644
#SPJ11
Describe and explain the trend in atomic a.radius within the group.Explain the difference between the size of b.the atoms and the size of the ions.
Answers
Answer:
A. The atomic radius increases (in a group) with the increasing atomic number. This is because atomic size generally increases from top to bottom within a group because the greater the number of protons means the greater amount of electrons. The amount of electrons in orbitals determine an atom's size/radius.
B. Since Group 1A (Alkali Metals) are metals, they tend to form cations. Cations are always smaller than their original atom because the greater positive charge from the nucleus closes in the space between it and the electrons, thus "shrinking" its size. This is why the ionic radii are smaller than the atomic radii of the same element.
I hoped this helped <3
Explanation: