Answers
The temperature at which the gas will increase to 1.37L is 744.6K.
How to calculate temperature?The temperature of a gas can be calculated using the Charles's law equation as follows;
V₁/T₁ = V₂/T₂
Where;
V₁ = initial volumeT₁ = initial temperatureV₂ = final volumeT₂ = final temperatureAccording to this question, a gas at 91°C occupies a volume of 0.67L. The temperature that the volume increase to 1.37L is:
0.67/364 = 1.37/T
0.00184 = 1.37/T
T = 1.37 ÷ 0.00184
T = 744.6K
Therefore, the temperature will increase to a value of 744.6K.
Learn more about temperature at: https://brainly.com/question/3491421
#SPJ1
Related Questions
What mass of H2 is needed to react with 8.75g oh O2 according to the following equation: O2(g)+H2(g)-->H2O(g)?
Answers
Answer:
1 mole Oxygen = 2 mole hydrogen0.21 mol oxygen = 0.54 mol hydrogenMass of hydrogen = moles molecularweight
Mass of hydrogen = 0.54 2 Mass of hydrogen = 1.08 grams.
Thus, for reacting with 8.75 grams of oxygen,
1.08 grams of hydrogen is required.
Explanation:
mark me brainliest!!
Why is respiration important to animals?
Respiration provides oxygen needed to release energy.
Respiration help reproduce new cells.
Respiration provides water to all cells in the body.
Respiration is used only for getting rid of waste product in the body.

Answers
\(\qquad \qquad\huge \underline{\boxed{\sf ᴀɴsᴡᴇʀ}}\)
The Correct choice is ~ A
Respiration provides oxygen needed to release energy.
Suppose that the microwave radiation has a wavelength of 12.4 cm . How many photons are required to heat 255 mL of coffee from 25.0 ∘C to 62.0 ∘C
Answers
Answer:
Explanation:
wavelength λ = 12.4 x 10⁻² m .
energy of one photon = h c / λ
= 6.6 x 10⁻³⁴ x 3 x 10⁸ / 12.4 x 10⁻²
= 1.6 x 10⁻²⁴ J .
Let density of coffee be equal to density of water .
mass of coffee = 255 x 1 = 255 g
heat required to heat up coffee = mass x specific heat x rise in temp
= 255 x 4.18 x ( 62-25 )
= 39438.3 J .
No of photons required = heat energy required / energy of one photon
= 39438.3 / 1.6 x 10⁻²⁴
= 24649 x 10²⁴
= 24.65 x 10²⁷ .
Phosphorus-32 has a half life of 14.0 days. A 40.0g sample is being shipped. It takes 27 days to arrive, how much P-32 remains?
Answers
The mass of P-32 remaining after 27 days, if the initial mass is 40g is 10.5 g.
What is half life of a radioactive element?
The half-life of a radioactive isotope is the amount of time it takes for one-half of the radioactive isotope to decay.
The half-life of a specific radioactive isotope is constant; it is unaffected by conditions and is independent of the initial amount of that isotope.
The number of half lives in 27 days;
n = 27 days/14 days
n = 1.929
The mass of P-32 remaining after 27 days, if the initial mass is 40g;
mass remaining = 40 g / (2^1.929)
mass remaining = 40 g / 3.808
mass remaining = 10.5 g
Thus, the mass of P-32 remaining after 27 days, if the initial mass is 40g is 10.5 g.
Learn more about half life here: https://brainly.com/question/2320811
#SPJ1
N
01H
H
The property of water shown allows it to-
A freeze faster than it boils due to sharing metallic bonds
B. support floating objects due to forces between covalent bonds
C remain stable due to electrons forming ionic bonds
D. be both cohesive and adhesive due to hydrogen bonds

Answers
Answer:
D
Explanation:
The special property of water is that it is able to be cohesive and adhesive due to their hydrogen bonds
which is the graph of the function g(x) = f(-x)
Answers
To graph the function g(x) = f(-x), you can start with the graph of f(x) and then reflect it about the y-axis.
What is a graph of the function g(x) = f(-x)?To find the graph of the function g(x) = f(-x), we can start with the graph of the function f(x) and then reflect it about the y-axis.
If the graph of f(x) is symmetric with respect to the y-axis, meaning it is unchanged when reflected, then g(x) = f(-x) will have the same graph as f(x).
However, if the graph of f(x) is not symmetric with respect to the y-axis, then g(x) = f(-x) will be a reflection of f(x) about the y-axis.
In either case, the resulting graph of g(x) = f(-x) will be symmetric with respect to the y-axis.
Learn more about the graph of functions at: https://brainly.com/question/17089414
#SPJ1

Who has course hero?I really need the “lion king…Ecology science.” Answer key so I can print it.It wont let me print mines.
Answers
Note that the above prompt on Ecology draws it's analysis form a well known story which have been told visually called "The Lion King".
The answers are:
1) Biotic Factors, simply put are living things
2 examples of things from Lion King Introduction are:
3) Abiotic factors are Non -living things.
4) Examples from the introduction are:
MountainWaterDirt5) the symbiotic relationship is called: commensalism.
What is commensalism?Long-term biological interactions known as commensalism occur when individuals of one species benefit while those of the other species suffer neither advantages nor harm.
Ecology which is the study of the environment, allows a person to comprehend how different types of creatures coexist in various kinds of physical settings.
Learn more about Ecology at:
https://brainly.com/question/30429252
#SPJ1
Full Question:
Although part of your question is missing, you might be referring to this full question:
1) What is Biotic Factors
2) List three biotic factors from the Lion King introduction
3) What is Abiotic factors?
4) List three Abiotic factors from the Lion King introduction
5) The bird riding on the tusks of the elephant feed on insects the elephant stirs up. What kind of symbiotic relationship exists between the two?
1. If liquids can be separated into layers of density, could gases also be separated? Explain your reasoning.
Answers
Hey there! I'll try to provide you with my best answer.
Answer: The main reason why it probably wouldn't be seperated is because the molecules in gas are much farther away from each other. This created a huge gap within the two states so sometimes it's likely to be not seperated. But it solely depends on the sort of gas is being used. If they have higher density difference then it is possible to be seperated.
If air is included as gas then yes, because there is heavy and light air. The heavy air is the cold air which you feel at night. Meanwhile the light air the warm air which rises in the sky during day light. Both of them are seperated because of their densities. Or atleast they are seperated into layers themselves. For example: this slightly cold and hot air is felt when your nearby a camp fire.
Hope it made sense.
In liquid the particles are a little far away from each other but they have a powerful attractive force in between them by which we can separate them into layers of density
On the other hand in gas the particles are too far apart from each other so we can't separate them into layers of density.
There are 5states of matter
SolidLiquidGasBose-Einstein CondensationPlasmaDone
How many grams of copper are in 239.45 grams of copper II phosphate? Please explain how you got the answer!!
Answers
The mass of copper in 239.45 grams of copper(II) phosphate, Cu₃(PO₄)₂ is 119.93 grams
How to determine the mass of copperThe mass of copper in 239.45 grams of copper(II) phosphate, Cu₃(PO₄)₂ can be obtained as follow:
1 mole of Cu₃(PO₄)₂ = (63.55 × 3) + 2[31 + (16× 4)] = 380.65 g/molMass of copper in 1 mole of Cu₃(PO₄)₂ = 63.55 × 3 = 190.65 gFrom the above data,
380.65 grams of Cu₃(PO₄)₂ contains 190.65 grams of copper.
Therefore,
239.45 grams of Cu₃(PO₄)₂ will contain = (239.45 × 190.65) / 380.65
239.45 grams of Cu₃(PO₄)₂ will contain = 119.93 grams of copper
Thus, we can conclude that the mass of copper is 119.93 grams
Learn more about mass composition:
brainly.com/question/9274824
#SPJ1
How much potassium nitrate could be dissolved into 2L of water
Answers
Answer:
About 110 g.
Your tool of choice here will be the solubility graph for potassium nitrate, KNO3, in water.
Answer:
613
Explanation:
search up that question at coursehero.com...these questions were originally made there, with the answers
A compound, C11H12O2, has an IR spectrum showing a peak at 1710 cm-1. Its 1H NMR spectrum has peaks at delta 1.3 (3 H, triplet), 4.3 (2 H, quartet), 6.5 (1 H, doublet), 7.4-7.6 (5 H, multiplet), and 7.7 (1 H, doublet).
Required:
Draw its structure below.
Answers
Answer:
Ethyl cinnamate
Explanation:
For this question, we have to start with the IR info. If we have a peak at 1710 this indicates the presence of a carbonyl group in the molecule (C=O). Additionally, if we calculate the I.H.D (index of hydrogen deficiency), we will have a value of "6". We already know that we have a C=O group, so, this counts for 1 of the 6 additionally, we can have a benzene ring so, this counts for 4, so far we have 5. Finally, we will have a double bond outside of benzene and we will have a total of 6, so:
Benzene: 4
Carbonyl group: 1
Double bond: 1
For a total of six (that fits with the I.H.D calculation). So, so far we know that we have a benzene ring, a double bond, and a carbonyl group. In the formula we have 2 oxygens, therefore we can have a carboxylic acid or an ester. In this case, the IR info doesn't give any additional info, so our best option is the ester group.
The 1H NMR info give is:
Signal A= 1.3 (3 H, triplet)
Signal B= 4.3 (2 H, quartet)
Singal C= 6.5 (1 H, doublet)
Signal D= 7.4-7.6 (5 H, multiplet)
Signal E= 7.7 (1 H, doublet)
The molecule that fits with this NMH spectrum and the info given by the I.H.D is "ethyl cinnamate".
See figure 1
I hope it helps!

The temperature inside my refrigerator is about 40 Celsius. That temperature in Kelvin is K.
I place a balloon in my fridge that initially has a temperature of 220 C. This is K.
If the original volume of the balloon is 0.5 liters, what will be the volume of the balloon when it is fully cooled by my refrigerator? liters. (Round to two decimal places)

Answers
Substituting the given values, we have (0.5 L) / (220 + 273.15 K) = V₂ / (313.15 K).Solving for V₂, we get V₂ = (0.5 L) * (313.15 K) / (220 + 273.15 K).
Calculating this expression, the volume of the balloon when fully cooled by your refrigerator would be approximately 0.38 liters when rounded to two decimal places.To convert Celsius to Kelvin, we need to add 273.15 to the Celsius temperature. Therefore, the temperature inside your refrigerator of 40 degrees Celsius is equivalent to 313.15 Kelvin.Now, let's consider the ideal gas law, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.Since the number of moles and pressure remain constant, we can write the equation as V₁/T₁ = V₂/T₂, where V₁ is the initial volume of the balloon, T₁ is the initial temperature, V₂ is the final volume, and T₂ is the final temperature.
for such more questions on values
https://brainly.com/question/27964828
#SPJ8
When dinitrogen pentoxide, N₂O5, a white solid, is heated, it decomposes to nitrogen dioxide and oxygen. How many grams of oxygen can be formed from the decomposition of 2.3 g of N₂05?
2N₂05 (s)—>4NO₂(g) + O₂(g)

Answers
The mass of the oxygen that can be obtained by the use of the stoichiometry of the reaction is 0.35 g.
How is stoichiometry applied?Stoichiometry is applied in chemistry to calculate the quantities of reactants and products involved in a chemical reaction. It involves using the balanced chemical equation of the reaction to determine the mole ratios of reactants and products, and then using these ratios to calculate the amount of one substance that is required to produce a given amount of another substance.
We know that;
Moles of N2O5 = 2.3g/108 g/mol
= 0.021 moles
2 moles of N2O5 would produce 1 moles of O2
0.021 moles would produce 0.021 * 1/2
= 0.011 moles
Mass of O2 = 0.011 moles * 32 g/mol
= 0.35 g
Learn more about moles:https://brainly.com/question/26416088
#SPJ1
Convert 3.0 cups/lb to L/kg
Answers
Answer:
1.56476
Explanation:
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
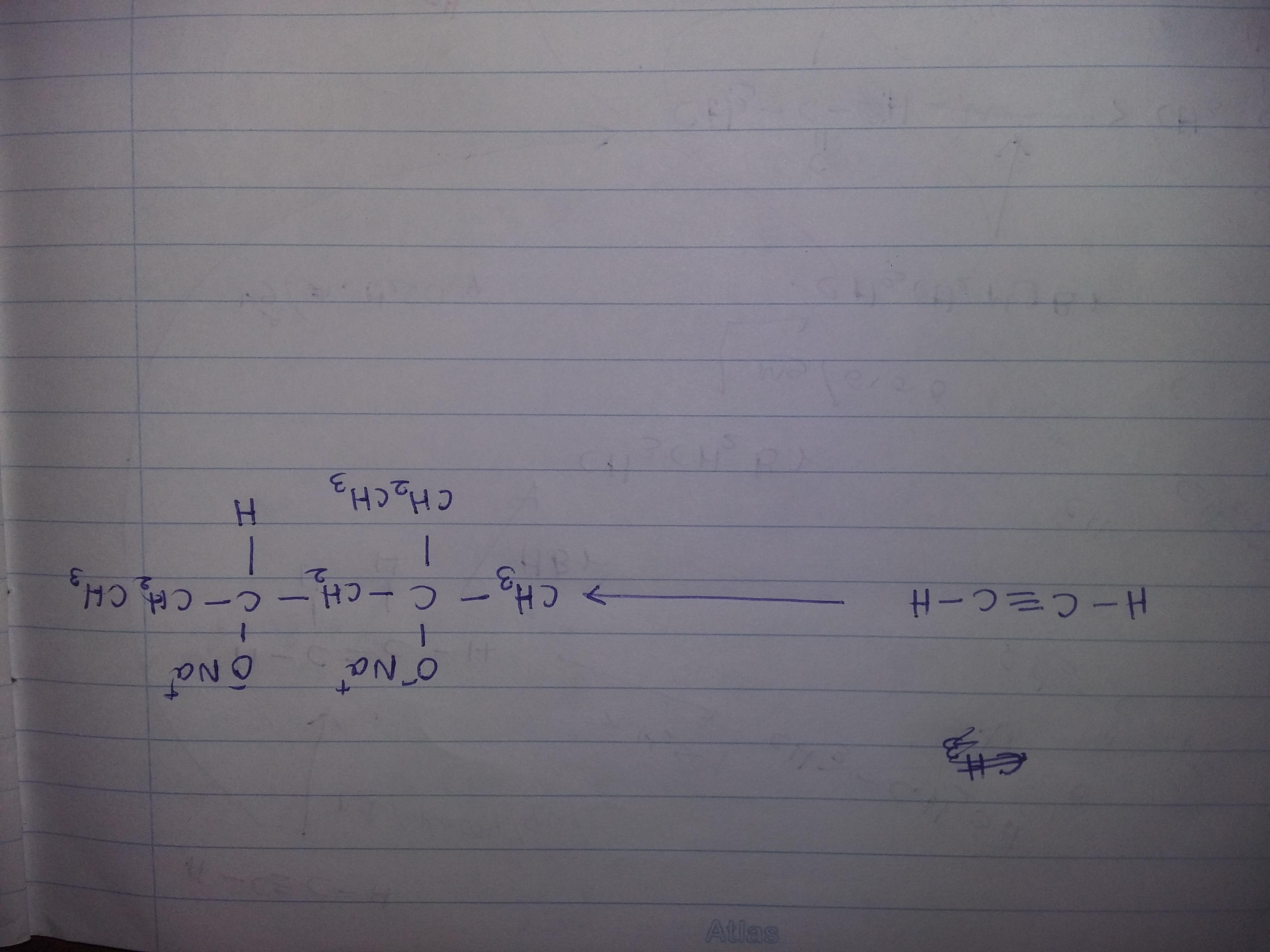
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
Plsss help im so confusing

Answers
Answer:
Refer to the picture. I managed to solve a few.

When 50 ml of 1.5 M HCl is added to 100 ml of 1.5 M sodium hydroxide solution, temperature
initially was 21.3 C and fainaly raised to 23.4 C, What is kj of heat produced? *
A)4643.2
mol
B)1102.9
mol
C)2211.05
mol
D)12381.86
mol
Answers
Answer:
Q = -1.318 KJ
Explanation:
We will use the assumption that this solution acts like water and thus we will use the specific heat capacity of water and when converting from mL to g, we will use the conversion like we do for water.
We are told that 50 mL of HCl reacts with 100 mL of NaOH.
Thus total mass; m = 50 + 100 = 150 mL
Converting to grams gives 150 g since we have assumed that the solution behaves like water.
We are given;
Initial temperature; T_i = 21.3° C
Final temperature; T_f = 23.4° C
ΔT = 23.4 - 21.3
ΔT = 2.1°C
Formula for quantity of heat is;
Q = mcΔT
c is specific heat capacity.
We will use c = 4.184 J/g°C since the solution is assumed to behave like water.
Thus;
Q = -(150 × 2.1 × 4.184)
Q = -1317.96 J
Negative sign is used because temperature was raised and thus reaction is exothermic.
Approximation to KJ gives; -1.318 KJ
Reversible chemical reactions contain a double reaction _____ in the chemical equation
Answers
Answer
Reversible chemical reactions contain a double reaction arrow, one pointing in each direction in the chemical equation.
Examples of such reaction is shown below:
\(A+B\rightleftarrows C+D\)Sound waves can travel across outer space true or false
Answers
Answer:
False
Explanation:
sounds waves can't travel so far
Explanation: Scientist thinks that there is actually sound in space it’s just that the human ear can’t hear it.
HURRY PLEASE!! A student builds a model of a town. The model's scale in 1:48. Fill in the missing
information in the table.
Distance between
in real life
in model
School and library
18.0 m
0375m 0
Grocery store and city hall
Select
14
26 m
Post office and park
9.6 m
30 m
MIT
864 m
Answers
School and store
48
Waves conduct energy through
Answers
(01.01 LC)What is the body of scientific knowledge based on?
Guesses
Mysteries
Observations
Opinions
Answers
The body of scientific knowledge is based on different Observations (Option C).
What does observations mean in the scientific method?Observations in the scientific method are fundamental because it is the first step to raising scientific questions that may be explained through plausible hypotheses. Subsequently, hypotheses must be tested by experimental procedures.
In conclusion, the body of scientific knowledge is based on different Observations (Option C).
Learn more about observations in the scientific method here:
https://brainly.com/question/2505873
#SPJ1
2. XC12 is the chloride of metal X. The formulae of its sulphate is
Answers
Answer:
XSO₄
Explanation:
XCl₂ is the chloride of metal X. The sum of the charges of the cation and the anion must be zero because the salt is electrically neutral. The charge of the cation of X is:
1 × X + 2 × Cl = 0
1 × X + 2 × (-1) = 0
X = +2
X has a charge +2 and sulphate (SO₄²⁻) a charge -2. The neutral salt they form is XSO₄.
Sarah is investigating the transfer of energy in the
form of heat. One process that transfers energy
between two objects is conduction. Which statement
is not true about conduction?
A. It involves a transfer of energy in the form of heat.
B. It can transfer energy through empty space.
C. It requires direct contact between the objects.
D. It results in a change of temperature in both
objects.
Answers
Answer:
B
Explanation:
In order for any conduction to take place it needs to go through a solid. Therefore it cannot travel through empty space
Answer:
b
Explanation:
What sample at STP has the same number of molecules as 5 L of NO2
Answers
Answer:
5l NO
2
at STP
No. of molecules=
22.4
5
mol=
22.4
5
×N
A
molecules
A) 5ℊ of H
2
(g)
No. of moles=
2
5
mol=
2
5
×N
A
molecules
B) 5l of CH
4
(g)
No. of moles of CH
4
=
22.4
5
mol=
22.4
5
N
A
molecules
C) 5 mol of O
2
=5N
A
O
2
molecules
D) 5×10
23
molecules of CO
2
(g)
Molecules of 5l NO
2
(g) at STP=5l of CH
4
(g) molecules at STP
Therefore, option B is correct.
Was this answer helpful?
Practice It! Which statement below is not a testable question? O How do you measure volume of an irregular shaped object? O Which chocolate melts fastest, dark or milk chocolate? O Do ants prefer sugar or salt? O Does mass affect how far a ball rolls?
Answers
The statement "Does mass affect how far a ball rolls?" is not a testable question as mass doesn't affect the speed directly.
How do you measure the volume of an irregular shaped object?When a solid object, like a stone, has an irregular shape, its volume can be determined by two methods. Measuring cylinder can be used as one method while using an overflow jar is another method.
Displacement of water or other liquids in a measuring cylinder determines the volume of irregular solid things that are heavier or lighter than water, including those that are soluble in water.
Which chocolate melts fastest, dark or milk chocolate?Milk chocolate takes longer to melt than dark chocolate.
Because dark chocolate has less fat than milk chocolate. Dark chocolate melts more quickly because the cocoa butter in it is crushed more finely.
More milk solids are found in milk chocolate, which raises the chocolate's melting point and increases its resistance to melting.
Do ants prefer sugar or salt?The researchers found that herbivorous or omnivorous species that were more than 10-100 kilometers (6-60 miles) from the ocean preferred salt over sugar.
They found out that the farther inland, the greater the preference for salt, by simply counting the ant species attracted to cotton balls soaked in sucrose (table sugar) or salt solutions.
While carnivorous ants showed little preference for salt over sugar, those that lived primarily on green plants showed a larger preference for salt than those that lived among the decomposing leaves of the forest floor.
Thus, all the other statements are testable except "Does mass affect how far a ball rolls?" as mass doesn't affect the speed directly, and hence we can't test it by a simple ball rolling experiment.
Learn more about mass:
https://brainly.com/question/19385703
#SPJ9
What does Chronic Disorder mean?
Answers
Answer:
Chronic diseases are defined broadly as conditions that last 1 year or more and require ongoing medical attention or limit activities of daily living or both. Chronic diseases such as heart disease, cancer, and diabetes
Answer:
conditions that last 1 year or more and require ongoing medical attention or limit activities of daily living
Explanation:
What volume of 12 M NaOH and 2 M NaOH should be mixed to get 2 litres of 9 M NaOH solution?
Answers
2/5lit.. of 2M NaOH
) A 3.50 gram sample of a hydrate of copper sulfate yields 2.10 g of anhydrous copper (II) sulfate.a. Determine the mass percent of water in the hydrate.
Answers
The mass percent is described by the following equation:
\(Mass\%(H_2O)=\frac{MassH_2O}{MassCuSO_4(H_2O)_x}\times100\%\)\(Mass\%(H_2O)=\frac{MassCuSO_4(H_2O)_x-MassCuSO_4}{MassCuSO_4(H_2O)_x}\times100\%\)CuSO4 corresponds to anhydrous copper (II) sulfate
CuSO4(H2O)x will be hydrate of copper sulfate
H2O corresponds to the water. The mass of water as we saw in the equation is the total mass of hydrate of copper sulfate minus the mass of anhydrous copper (II) sulfate
So, we can replace the known masses:
\(Mass\%(H_2O)=\frac{3.50g-2.10g}{3.50g}\times100\%\)\(Mass\%(H_2O)=40.0\%\)Answer: The mass percent of water in the hydrate is 40.0%
use scientific notation to solve

Answers
Hope this helped :)