A hypothesis is best written as ?
Answers
Answer:
A good experimental hypothesis can be written as an if, then statement to establish cause and effect on the variables. If you make a change to the independent variable, then the dependent variable will respond
Related Questions
Can someone do this for me? Pleaseeeee I’m very busy.
The directions tell you what to do.

Answers
Answer:
in number 7 its Gas because the molecules are afloat and/or moving rapidly.
Explanation:
Write the correct abbreviation for each metric unit.
1) Kilogram __ 4) Milliliter __ 7) Kilometer __ 2) Meter 5) Millimeter __
8) Centimeter __ 3) Gram __ 6) Liter __ 9) Milligram __
Answers
The correct abbreviation for each metric unit is:
Kilogram - kg, Milliliter - ml, Kilometer- Km, Meter- m, Millimeter - mm, Centimeter - cm, Gram - g, Liter - L, and Milligram - mg.
What is the metric system?The metric system can be described as a system of measurement that succeeded the decimalized system based on the meter. Each of the fundamental dimensions can be expressed by a single base unit of measure.
For quantities derived from the base units of the system, units derived from the base units are used such as the square meter being the derived unit for the area, a quantity derived from length.
Metric units can be described as units based on the meter, gram, or second and decimal multiples or sub-multiples of these. The units of the International System of Units (SI). By extension, they involve units of electromagnetism from the CGS units and SI units systems.
Learn more about Metric units, here:
https://brainly.com/question/19483018
#SPJ1
A 25.0 mL sample of a solution of an unknown compound is titrated with a 0.115 M NaOH solution. The titration curve above was obtained with pH at equivalence point of around 8. The unknown compound is ________.
Answers
Answer:
Weak acid
Explanation:
A titration curve is a graphical description of the change in pH of the solution in the conical flask as the reagent is added from the burette. A titration curve can be plotted for the different kinds of acid and base titrations. The volume of the titrant is always plotted as the independent variable and the pH of the solution as the dependent variable. The equivalence point is read off from the titration curve. A titration curve is very important because it shows the pH at various points during the titration.
A weak acid/strong base titration leads to an equivalence point above 7. From the question, we were told that the pH at equivalence point lies around 8. Hence the unknown substance must be a weak acid.
The vapour pressure of water 12.3kPa at 300K. Calculate the vapour pressure of 1 molal solution of a non-volatile solute in it.
Answers
Answer:
ywhehehehehdgdudcdudcd uy dshdcvv
Explanation:
bdbdkzkzjzdkdkzdvdisisksjskakskskzbx vxydcdudschahahbshshs
a mixture of gases contains 5.10 g of n2, 2.83 g of h2, and 5.17 g of nh3. if the total pressure of the mixture is 2.35 atm, what is thepartial pressure of each compononet?
Answers
The partial pressure of N2 is 0.161 atm, the partial pressure of H2 is 1.16 atm, and the partial pressure of NH3 is 0.217 atm.
The partial pressure of each component in a mixture of gases can be calculated by dividing the number of moles of each gas by the total number of moles of all the gases in the mixture and then multiplying by the total pressure of the mixture.
First, we need to calculate the number of moles of each gas:
N2: 5.10 g / 28.02 g/mol = 0.181 mol
H2: 2.83 g / 2.02 g/mol = 1.40 mol
NH3: 5.17 g / 17.03 g/mol = 0.304 mol
Total number of moles: 0.181 mol + 1.40 mol + 0.304 mol = 2.885 mol
Next, we can calculate the partial pressure of each gas:
N2: 0.181 mol / 2.885 mol × 2.35 atm = 0.161 atm
H2: 1.40 mol / 2.885 mol × 2.35 atm = 1.16 atm
NH3: 0.304 mol / 2.885 mol × 2.35 atm = 0.217 atm
So, the partial pressure of N2 is 0.161 atm, the partial pressure of H2 is 1.16 atm, and the partial pressure of NH3 is 0.217 atm.
Learn more about partial pressure:
brainly.com/question/13199169
#SPJ4
To determine the molar mass of an unknown from the freezing point depression, the freezing points of both the pure solvent and the solution need to be measured.
Answers
Yes, to determine the molar mass of an unknown solute from the freezing point depression the freezing points of both the pure solvent and the solution need to be measured.
The depression of the freezing point is a and it is given by the following formula,
ΔT f = T°f - T f
Here T°f is the freezing point of pure solvent
T f is the freezing point of the solution made by adding non-volatile solute to solvent
The freezing point depression is directly proportional to the molality of the solution
T(f) ∝ m
T(f)= K f x m
Here K f is the proportionality constant.
Hence, to determine the molar mass of an unknown solute from the freezing point depression the freezing points of both the pure solvent and the solution need to be measured.
To know more about "freezing point depression", refer to the link given below:
https://brainly.com/question/2292439?referrer=searchResults
#SPJ4
A spring with a spring constant of 3N/m is stretched until extended by 1.4m. How much elastic potential energy is stored by the spring?
Give your answer to 2 decimal places
Answers
The energy that is stored in the spring is 2.94 J.
What is the elastic potential energy?We have to note that the elastic potential energy of the spring is the energy that has been stored in the spring and it is released to be able to do work once we pull the spring.
In this case we already know that;
W = 1/2Ke^2
W = work done
K = force constant
e = extension
Thus;
W = 0.5 * 3 * 1.4^2
W = 2.94 J
We can see that the stored energy for the spring in question is 2.94 J of energy stored.
Learn more about spring constant:https://brainly.com/question/14159361
#SPJ1
What does the pH stand for?
Answers
Answer: potential hydrogen
Explanation:
Potential hydrogen is what pH stands for. Based on the concentration of hydrogen ions (H+) in the solution, it determines the acidity or basicity (alkalinity) of a solution.
What is pH?A solution's pH, which ranges from 0 to 14, with 7 denoting neutrality, indicates how acidic or basic it is. It also represents the amount of hydrogen ions that are present.
The negative logarithm (base 10) of the hydrogen ion concentration, written as [H+], is used to calculate a solution's pH.
[H+] = 10-pH can be used to compute the hydrogen ion concentration in a solution, which is expressed in moles per litre (mol/L). For instance, a pH 4 solution has a hydrogen ion concentration of 10-4 mol/L, which is 10 times more than a pH 5 solution.
In several disciplines, including biology, chemistry, environmental science, and food science, the pH of a solution is significant. In biology, the blood pH normally ranges from 7.35 to 7.45, and the pH of the human body is tightly controlled to maintain a healthy balance. A shift in blood pH, also referred to as acidosis or alkalosis, can have detrimental effects on one's health.
Since pH has an impact on a substance's solubility and reactivity, it plays a significant role in many chemical reactions. For instance, many industrial processes, including the manufacture of fertilisers and paper, rely on controlling the pH of the used solutions. This is because the pH of a solution can impact the rate at which enzymes function.
To know more about potential hydrogen, visit:
https://brainly.com/question/13480308
#SPJ4
What volume of dichloromethane (ch2cl2) is produced when 149 liters of methane (ch4) react according to the following reaction? (all gases are at the same temperature and pressure. ) methane (ch4)(g) carbon tetrachloride(g) dichloromethane (ch2cl2)(g)'
Answers
The volume of dichloromethane \((CH_2Cl_2)\) produced when 149 liters of methane \((CH_4)\) react according to the given reaction is approximately 6.224 x \(10^5 J/K*m^3\).
The volume of dichloromethane \((CH_2Cl_2)\) produced when 149 liters of methane \((CH_4)\) react according to the given reaction is not immediately apparent from the reaction stoichiometry.
The balanced equation for the reaction between methane \((CH_4)\) and carbon tetrachloride (CCl4) to form dichloromethane \((CH_2Cl_2)\) and carbon dioxide (CO2) is:
\((CH_4)\) + \(CO_2\) → \((CH_2Cl_2)\) + \(CO_2\)
The balanced equation shows that 1 mole reacts with 1 mole of CCl4 to produce 1 mole of \((CH_2Cl_2)\) and 1 mole of \(CO_2\).
The volume of the gas can be calculated using the ideal gas law:
PV = nRT
To find the number of moles of gas, we can use the molecular masses of the reactants and products:
Molar mass of \((CH_4)\) = 16.04 g/mol
Molar mass of \(CCl_4\) = 89.9 g/mol
Molar mass of \((CH_2Cl_2)\) = 70.1 g/mol
Molar mass of \(CO_2\) = 44.01 g/mol
The number of moles of \((CH_4)\) can be calculated from the initial amount of gas:
149 L of CH4 = 149 x 16.04 g/mol = 2432 g
The number of moles of CCl4 can be calculated from the given volume:
149 L of \((CH_4)\) + \(CCl_4\) → \((CH_2Cl_2)\) + \(CO_2\)
The volume of the gas is given as 149 L, so the number of moles of \(CCl_4\) can be calculated as:
149 L = 149 x 89.9 g/mol = 13,277 g
The number of moles can be calculated from the given volume and the desired amount of product
149 L of \((CH_4)\) + \(CCl_4\) → \((CH_2Cl_2)\) + \(CO_2\)
149 L of \((CH_4)\) + \(CCl_4\) → 149 x 70.1 g/mol + 13,277 g x 1 mol/13.277 g = 43,691 g
V = nRT
V = 43,691 g x 8.314 J/mol·K = 364,617.5 J/K
1 J/K = 1/1000 L·K
Therefore, the volume of the gas is:
V = 364,617.5 J/K x (1/1000 L·K) = 3.646 x 10^4 L
substitute this value for V in the equation for the volume of \((CH_2Cl_2)\) :
PV = nRT
PV = 149 x 8.314 J/mol·K x (3.646 x \(10^4\) L)
PV = 6.224 x \(10^5 J/K*m^3\).
Therefore, The volume of dichloromethane \((CH_2Cl_2)\) produced when 149 liters of methane \((CH_4)\) react according to the given reaction is approximately 6.224 x \(10^5 J/K*m^3\).
Learn more about dichloromethane Visit: brainly.com/question/31080842
#SPJ4
The initial concentration of sodium oxalate, Na₂C₂O4 is 1.34 M. After 19.3 seconds its concentration is
0.276 M
(Triangle)Na₂C₂O4 =
Rate =
Answers
The rate of change of the concentration of Na₂C₂O4 is -0.0551 M/s.
To determine the rate of change of the concentration of sodium oxalate (Na₂C₂O4), we can use the rate equation:
Rate = (Δ[Na₂C₂O4]) / (Δt)
where Δ[Na₂C₂O4] represents the change in concentration of Na₂C₂O4 and Δt represents the change in time.
In this case, the initial concentration of Na₂C₂O4 is 1.34 M, and after 19.3 seconds, the concentration is 0.276 M.
Substituting the values into the rate equation, we have:
Rate = (0.276 M - 1.34 M) / (19.3 s - 0 s)
Rate = (-1.064 M) / (19.3 s)
Rate = -0.0551 M/s
Therefore, the rate of change of the concentration of Na₂C₂O4 is -0.0551 M/s.
The negative sign indicates that the concentration of Na₂C₂O4 is decreasing over time, as the reactant is being consumed in the reaction.
It's important to note that the rate of a reaction is influenced by various factors, such as the reaction mechanism, temperature, and presence of catalysts. The rate can be determined experimentally by measuring the change in concentration of a reactant or product over a specific time interval.
The given information allows us to calculate the rate of change for the specific reaction involving Na₂C₂O4. However, without additional information about the reaction, it is not possible to determine the exact nature or stoichiometry of the reaction, as well as any other reactants or products involved.
For more such questions on rate of change visit:
https://brainly.com/question/29000078
#SPJ8
What is the net ionic equation when agno3 and nh4cl are mixed?
Answers
The net ionic equation for the reaction between AgNO3 and NH4Cl is: Ag+(aq) + Cl-(aq) → AgCl(s)
When AgNO3 (silver nitrate) and NH4Cl (ammonium chloride) are mixed, a white precipitate of AgCl (silver chloride) is formed.
AgNO3 is a soluble salt that dissociates in water to form Ag+ and NO3- ions, while NH4Cl also dissociates in water to form NH4+ and Cl- ions. When these two solutions are mixed, the Ag+ ions react with the Cl- ions to form an insoluble precipitate of AgCl, which is a white solid. The ammonium ion (NH4+) and the nitrate ion (NO3-) are spectator ions and do not participate in the reaction.
The overall balanced chemical equation for the reaction is:
AgNO3(aq) + NH4Cl(aq) → AgCl(s) + NH4NO3(aq)
The net ionic equation shows only the species that are involved in the reaction and excludes spectator ions. Therefore, the net ionic equation for the reaction between AgNO3 and NH4Cl is:
Ag+(aq) + Cl-(aq) → AgCl(s)In the net ionic equation, the ammonium ion (NH4+) and the nitrate ion (NO3-) are not shown since they do not participate in the reaction.
To learn more about spectator ions Click here:
brainly.com/question/28913274
#SPJ4
SHOW WORK
Calculate the morality
10.0 g of sodium chloride is dissolved in 2.0 L of solution.
Answers
Determine if the following reaction is a redox reaction. Use evidence from the equation to explain your reasoning.

Answers
A redox reaction is a chemical reaction in which one or more of the reacting species undergoes oxidation and one or more undergoes reduction. An oxidizing agent is an element or compound that oxidizes another substance, while a reducing agent is an element or compound that reduces another substance.
The following reaction is a redox reaction based on the following evidence: 2Al + 3FeO → Al2O3 + 3Fe2+ In this reaction, Fe is being reduced because the FeO is changing to Fe2+. Additionally, the Al is being oxidized because it is losing electrons and forming Al2O3. Therefore, the reaction is a redox reaction. Let us take a look at the oxidation state of the elements in the given equation. Oxidation state of Al: (2) for the reactant and (3+) for the product. Oxidation state of Fe: (2+) for the reactant and (2+) for the product. Oxidation state of O: (-2) for the reactant and (-2) for the product. We can tell that oxidation is happening because of the increase in the oxidation state of Al from 2 to 3+. We can tell that reduction is happening because of the decrease in the oxidation state of Fe from 2+ to 2. As a result, the given equation is a redox reaction.For such more question on oxidizes
https://brainly.com/question/14041413
#SPJ8
What volume would 32.0g of NO¹² gas occupy at 3.12 ATM and 18.0°c? What volume would 32.0 g of nitrogen oxide gas occupy at 3.12 ATM and 18.0? Show working out
Answers
Answer:
THE VOLUME OF 32 g OF NO GAS AT 3.12 atm AND 18 °C IS 79.30 L
Explanation:
Mass = 32 g
Pressure = 3.12 atm
Temperature = 18 °C = 18 + 273 K= 291 K
Molar mass of gas NO = (14 + 16) = 30 g/mol
Gas constant = 0.82 L atm mol^-1 K^-1
Volume = unknown
Using PV = nRT where: number of moles = mass / molar mass
PV = m RT / MM
V = mRT / MM P
V = 32 *0.82 * 291 / 30 * 3.21
V = 7635.84 / 96.3
V = 79.30 L
The volume of 32 g NO gas at 3.21 atm and 18 °C is 79.30 L
What is the charge of an atom with and equal number of protons and electrons
Answers
Because they have an equal number of negative charges (electrons) and positive charges (protons). So, they will cancel out and the answer will be 0.
what is the orbital diagram for the valence electrons in a ground state atom of nitrogen?
Answers
An orbital diagram is a graphical representation of the arrangement of electrons within the orbitals of an atom or ion. It provides a visual depiction of the electron configuration, showing the distribution of electrons among different energy levels and orbitals.
The orbital diagram for the valence electrons in a ground-state atom of nitrogen can be represented as follows: N: 1s² 2s² 2p³.In this diagram, the "1s²" and "2s²" orbitals are filled with electrons, while the "2p³" orbital has three electrons occupying it. The "2p" orbital has three sub-orbitals, each of which can hold up to two electrons. In the case of nitrogen, two of the sub-orbitals are filled with one electron each, while the third sub-orbital has two electrons. This gives nitrogen a total of five valence electrons.
Learn more about orbital diagram here ;
https://brainly.com/question/28809808
#SPJ11
Calculate the number of moles of an ideal gas if it occupies 1750 dm3 under 125,000 pa at a temperature of 127 c. a. 0.21 moles b. 1.72 moles c. 546.88 moles d. 65.81 moles
Answers
The number of mole will be 65.81 mole.
An ideal gas would be one for which both the overall volume of the molecules and even the forces that exist between them are so negligible as to have no influence on the behavior of something like the gas.
Number of ideal gas can be calculated by using the formula:
PV = nRT
where, p is pressure, n is number of mole, R is gas constant and T is temperature.
Given data:
V= 1750 \(dm^{3}\) = 1750 L
P = 125,000 p = 1.2 atm
R = 0.082 L /mole kelvin
T = 273+127 = 400 K
Now, put the value of given data in above equation.
1.23atm x 1750L = n x 0.0820atm x Liter/ mole x kelvin x 400K
n = 65.81 mole.
Therefore, the number of mole will be 65.81 mole
To know more about mole
https://brainly.com/question/21050624
#SPJ4
Can you please help me with this!

Answers
How many moles of copper would be needed to make one mole of cu2o?
Answers
Answer:
Explanation:
You can view more details on each measurement unit: molecular weight of Copper(I) Oxide or grams The molecular formula for Copper(I) Oxide is Cu2O. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Copper(I) Oxide, or 143.0914 grams.
Which term is defined as the sum of protons and neutrons in an atom?.
Answers
Answer:
mass number
Explanation:
yeah mass number is the total of protons and neutrons
for example carbon has 12 proton and 12 neutrons therefore it's mass number is 24
i hope this is what you're looking for
What is the percentage of Calcium in CaC2?
O 63 %
O 77%
O 23%
O 37 %
Answers
Answer:
23percent
Explanation:
correct this or not?
how many rings are present in c14h19io3? this compound consumes 3 mol of h2 on catalytic hydrogenation. enter your answer in the provided box. ring(s)
Answers
The compound C14H19IO3 has one ring. This can be determined by analyzing its molecular structure.
The presence of a ring can be identified by examining the connectivity of atoms in the compound. In this case, there is one cyclic structure present in the compound.
It is worth noting that the number of hydrogen molecules consumed during catalytic hydrogenation is not directly related to the number of rings in the compound.
The reaction of the compound with 3 mol of H2 indicates the number of moles of hydrogen gas required for the reaction, which is independent of the presence or absence of rings.
to know more about ring structure visit:
https://brainly.com/question/32287115
#SPJ11
True or false the main factor affecting the speed of sound in air is the pressure of the air
Answers
Answer:
FALSE
Explanation:
The temperature of the air has a significant impact on the speed of sound in that medium. The square root of the absolute temperature of the air determines the speed of sound in that medium. The speed of sound rises along with temperature.
The speed of sound is also influenced by pressure, however this influence is minimal. The speed of sound will rise with increasing pressure and decrease with decreasing pressure, however the effect is quite tiny in comparison to the influence of temperature.
In conclusion, air temperature has a significant impact on the speed of sound in air, with pressure having a marginally smaller impact.
write anodic and cathodic reaction involve in fuel cell
Answers
Cell reactions in a fuel cell:
Oxidation at the anode (-): Oxidation of hydrogen gas to water occurs at the anode. Reduction at the cathode (+): Reduction of oxygen gas to OH ions occurs at the cathode.
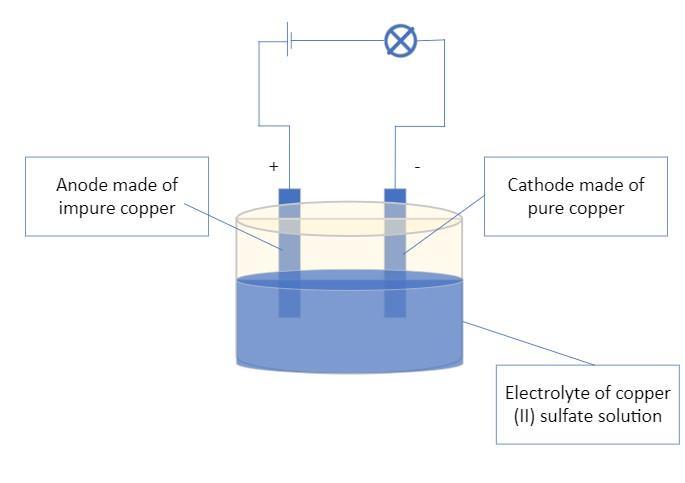
Can any person help me for this problem? Anyone's welcome

Answers
Answer:
The anode made of the impure copper
The cathode made of pure copper
The electrolyte of copper (II) sulfate CuSO₄ solution
The silver impurities at the anode due to their high tendency of accepting electrons and being a stronger reducing agent than either copper or zinc will remain relatively in place and relatively stable and will not actively take part in the oxidation reaction taking place at the anode
The zinc impurities will be the first element of the three metals to give up electrons and go into the solution as zinc ions due to their high tendency to loan out two electrons and become oxidized into Zn²⁺ ions
The drawing of the electrolytic cell created with Microsoft Visio is attached
Explanation:
Which property of matter is conserved in chemical reactions and shown by balanced equations?
Answers
The property of matter that is conserved in chemical reactions and shown by balanced equations is known as the Law of Conservation of Mass. According to this law, mass can neither be created nor destroyed in a chemical reaction; it can only be transformed from one form to another.For instance, when two substances are combined, they react and form a new substance.
The products that are formed contain the same number of atoms as the reactants, but in different configurations. To keep track of the number of atoms on either side of the equation, we use coefficients, which indicate the number of molecules or atoms of each substance in the reaction. However, when a chemical equation is written, it must adhere to the law of conservation of mass.The law of conservation of mass is critical in chemical reactions because it ensures that the amount of reactants that go into a reaction equals the amount of products that come out of it. This means that the total mass of reactants must equal the total mass of the products. As a result, the balanced chemical equation must reflect this law.For example, consider the reaction between hydrogen gas and oxygen gas, which forms water. The balanced chemical equation is as follows:2H2 + O2 → 2H2OIn this reaction, two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water. The coefficients in the balanced chemical equation indicate that two molecules of hydrogen and one molecule of oxygen combine to form two molecules of water, obeying the law of conservation of mass.In conclusion, the Law of Conservation of Mass is a fundamental principle in chemistry that is used to balance chemical equations. It is critical in chemical reactions because it ensures that the total mass of reactants equals the total mass of products, allowing scientists to accurately predict the outcome of a chemical reaction.For such more question on chemical reaction
https://brainly.com/question/11231920
#SPJ8
Rate the relative polarity of each solvent. List them from least to most polar!
THANK YOU:)
P.s. we re assuming that (alcohol) is isopropyl
Answers
The relative polarity of solvents can be determined by comparing their dielectric constants, which measure the solvent's ability to separate and stabilize charged particles. The higher the dielectric constant, the more polar the solvent.
We can list the solvents from least to most polar as follows: 1. Hexane - Hexane is a nonpolar solvent with a dielectric constant of 1.9, making it the least polar of the solvents listed. 2. Ethyl acetate - Ethyl acetate is a polar aprotic solvent with a dielectric constant of 6.0. While it is more polar than hexane, it is still relatively nonpolar compared to the other solvents on this list. 3. Acetone - Acetone is a polar aprotic solvent with a dielectric constant of 20.7. It is significantly more polar than ethyl acetate and is often used in organic chemistry reactions as a solvent and a reagent. 4. Isopropyl alcohol - Isopropyl alcohol (also known as rubbing alcohol) is a polar protic solvent with a dielectric constant of 20.0. It is more polar than acetone due to its ability to form hydrogen bonds, which makes it a better solvent for ionic compounds. 5. Water - Water is the most polar of the solvents listed, with a dielectric constant of 78.5. Its high polarity is due to its ability to form strong hydrogen bonds, which makes it an excellent solvent for polar and ionic compounds.
The relative polarity of solvents can have a significant impact on their ability to dissolve and stabilize different types of compounds. By understanding the properties of different solvents, chemists can choose the most appropriate solvent for a given reaction or separation process.
To know more about polarity visit :-
https://brainly.com/question/30397146
#SPJ11
6. Glucose, C,H,O, is a kind of sugar
molecule. How many atoms are in a
molecule of glucose?
A.6
B. 12
C. 18
D. 24
Answers
What is the volume of a container that contains 12.6 grams of nitrogen at 25°C and 1100 kPa?
Answers
Answer:
1.0 L
Explanation:
Use the ideal gas law PV=nRT where P is pressure = 1100 kPa, V is the volume you are calculating, n is the number of moles which can be calculated by dimensional analysis and the periodic table, R is the ideal gas constant in this case R=8.314 kPa L/K mol, and T is the temperature in kelvins. See attached image for work through. 1.0 L is the answer with two sig figs.

calculate the wavelength of light emitted from a hydrogen atom when it undergoes a transition from the n
Answers
When the electron in a hydrogen atom transitions from n, light with a wavelength of 486 nm is released.
Radio waves, light waves, and infrared (thermal) waves are examples of electromagnetic radiation that produce distinctive patterns as they move across space. Each wave is unique in its length and shape. The wavelength is the separation between peaks (high points). Therefore, when the electron in a hydrogen atom transitions from n, light with a wavelength of 486 nm is released. Radio waves, light waves, and infrared (thermal) waves are examples of electromagnetic radiation that produce distinctive patterns as they move across space. Each wave is unique in its length and shape.
To learn more about wavelength :
https://brainly.com/question/29807666
#SPJ4