Answers
The sample is undergoing chemical change.
When one chemical material changes into one or more others, as when iron rusts, this is referred to as a chemical change.
There are five signs that shows us that the object is undergoing chemical change-
1. Change of colour
2. Change of temperature
3. Gas evolution
4. Precipitate formation
5. Production of smell
Therefore According to the given question the sample is giving off a solid precipitate of black colour. Also, there is evolution of gas so the given sample is undergoing a chemical change.
Additionally, this is a type of Decomposition reaction where in the gas evolved is a Carbon dioxide. Also the given sample is a Compound because two products are formed.
Hence, it is a chemical change.
To learn more about chemical change refer- https://brainly.com/question/1222323
#SPJ9
Related Questions
A solution that is neutral has a pH of:
0
14
10
1
7
Answers
CuBr2 percent composition
Answers
The percent composition of CuBr₂ is approximately 28.46% of Cu and 71.54% of Br.
To determine the percent composition of CuBr₂ (copper(II) bromide), we need to calculate the mass of each element in the compound and then divide it by the molar mass of the entire compound.
The molar mass of CuBr₂ can be calculated by adding up the atomic masses of copper (Cu) and bromine (Br) in the compound. The atomic masses of Cu and Br are approximately 63.55 g/mol and 79.90 g/mol, respectively.
Molar mass of CuBr₂ = (63.55 g/mol) + 2(79.90 g/mol) = 223.35 g/mol
Now, let's calculate the percent composition of each element in CuBr₂:
Percent composition of copper (Cu):
Mass of Cu = (63.55 g/mol) / 223.35 g/mol × 100% ≈ 28.46%
Percent composition of bromine (Br):
Mass of Br = 2(79.90 g/mol) / 223.35 g/mol × 100% ≈ 71.54%
Therefore, the percent composition of CuBr₂ is approximately:
- Copper (Cu): 28.46%
- Bromine (Br): 71.54%
These values represent the relative mass percentages of copper and bromine in the compound CuBr₂.
for more such questions on composition
https://brainly.com/question/28250237
#SPJ8
What mass of carbon dioxide is produced by the complete combustion of 48.0 g of hexane?
Answers
The mass of carbon dioxide produced by the complete combustion of 48.0g of hexane is 147.35g.
How to calculate mass?The mass of a substance can be calculated by using the stoichiometry approach.
The balanced equation of the complete combustion of hexane is as follows:
2C6H14(g) + 19O2(g) → 12CO2(g) + 14H2O(g)
48g of hexane to moles is calculated as:
48g ÷ 86g/mol = 0.56 mol
2 moles of hexane produces 12 moles of CO2
0.56 mol of hexane will produce 0.56 × 12/2 = 3.35 mol
Next, we convert 3.35 moles of CO2 to mass as follows:
3.35 moles × 44g/mol = 147.35g
Therefore, the mass of carbon dioxide produced by the complete combustion of 48.0g of hexane is 147.35g.
Learn more about mass at: https://brainly.com/question/19694949
#SPJ1
How can knowledge of separating mixtures help you in daily life and within society? Answer below.
Answers
Answer:
I can say that knowledge of separating mixtures can help us in daily life and within society in the following ways:
1. Purification of water: Separation techniques are used to purify water for drinking and industrial purposes. It is essential to remove impurities from water to prevent diseases.
2. Recycling: Separation techniques are used to separate materials for recycling. This helps reduce the amount of waste in landfills and helps conserve resources.
3. Food industry: Separation techniques are used in the food industry to separate unwanted particles from food products. This helps ensure that the food we eat is safe and free from contaminants.
4. Medicine: Separation techniques are used in the pharmaceutical industry to separate and purify chemicals for use in medicine. This helps ensure that medicines are safe and effective.
5. Environmental protection: Separation techniques are used to remove pollutants from the environment. This helps protect our natural resources and prevent pollution-related health problems.
6. Oil and gas industry: Separation techniques are used to separate crude oil and natural gas into their various components. This helps in the production of energy and other useful products.
In summary, knowledge of separating mixtures is essential in our daily lives and within society. It helps ensure that we have access to safe and clean water, food, medicine, and energy, and also helps protect the environment.
Explanation:
Determine which physical conditions are necessary to support nuclear fusion and formation of stars.

Answers
Answer:
The correct approach will be "Increased gravitational attraction".
Explanation:
The increased gravitational attraction seems to be the natural or physical phenomenon that is required to promote nuclear reactions including star formation. This is much more important to establish stars at different temperatures (lower) and greater magnetic pull as well as nuclear fusion tends to happen.What are positively radioactive rays called
Answers
Answer:
False
Explanation:
Hope I helped! Love, Josie.

Increased industrialization has caused a rise in harmful acid rain precipitation that affects plant and marine life. A sample of acid rain has a proton concentration 10,000 times greater than pure water and more than 100,000 times greater than seawater. What is the approximate pH of this sample?
Answers
Answer:
pH of the sample of acid rain is 3.
Explanation:
Pure water has a theoretical pH of 7.00. As pH = -log [H⁺], [H⁺] = 1x10⁻⁷M
Now, the sample of acid rain has a proton concentration 10,000 times greater than pure water. That is:
[H⁺] = 10,000 * 1x10⁻⁷M = 1x10⁻³M
The pH of this sample is:
pH = -log 1x10⁻³M
pH = 3or which of the following molecules would the intermolecular forces be influenced mainly by hydrogen bonding? BrF5 C2H5OH H2S CH3NH INFLUENCE OR NOT
Answers
The molecules that would have the intermolecular forces be influenced mainly by the hydrogen bonding is C₂H₅OH , CH₃NH₂.
The Hydrogen bonding is the special type of the dipole-dipole attraction between the molecules, but not the covalent bond to the hydrogen atom. It will results from the attractive force in between the hydrogen atom that are covalently bonded to the more electronegative atom such as the N, O, or F atom and the another very electronegative atom. The hydrogen bonding, is the interaction that involves a hydrogen atom that is located in between a pair of the other atoms having the high affinity for the electrons
Thus, C₂H₅OH , CH₃NH₂ both molecules influenced by the hydrogen bonding.
To learn more about hydrogen bonding here
https://brainly.com/question/12514139
#SPJ4
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
The number of electrons in a natural atom having atomic number of 48 in mass number of 114 is:A)48B)114C)66D)162
Answers
In neutral atoms, the number of protons, the number of electrons and the atomic number will be all equal to each other, therefore if an atom has 48 of atomic number and it is neutral, it will also have 48 electrons and 48 protons
What is the molar mass of fluorapatite (Ca5(PO4)3F)?
Answers
Answer: 504.3
Explanation: Add the respective weights of each of the elements in the compound together.
c6h12o6 + 6o2 > 6 co2 + 6h2o
How many molecules of C6h12o6 are needed to produce 18 molecules of co2?
A: 3
B: 9
C: 12
D: 18
Answers
Answer:
A or 3 would be the right answer
In an experiment, 0.100 mol H2 and 0.100 mol I2 are mixed in a 1.00-L container and the reaction forms HI.
If Kc = 50.0 for this reaction, what is the equilibrium concentration of HI?
I2(g) + H2(g) → 2 HI(g)
Answers
Answer: HI = 0.1559
Explanation:


Which one of these are a physical property? Select all that apply. a. Luster (shininess) Conducts Electricity Reactivity with water d. Temperature b. 0 0
Answers
Part 1: Research Include important facts found through your research. Include the answers to questions 1-3. Part 2: Activity Include the answers to questions 1-4. Submission Requirements: The student should submit a presentation created with a slideshow or presentation program of their choice. The presentation should include important facts obtained through research, references, images, data, graphs, and answers to the questions in Part 1 and Part 2.
Answers
Answer:
I dont have time for the presentation so ill just give the answers to the questions you can just copy and paste them into a gooogle slides
Explanation:
1. According to the law of superposition, if the terrain was undisturbed, the layer E would be the youngest and the layer A would be the oldest.
2. The law of horizontality states that sediment deposited into water will settle at the bottom in flat, horizontal layers. This would make us expect that we could find layer D in sector 3. To explain the absence, if none of the stratum D species lived there, there would be no stratum D fossils there.
3. The law of faunal and floral succession suggests that the graphic did not go through any geologic processes after deposition.
4. According to the law of cross-cutting relationships, Layer A is the oldest, and Magma F is the youngest.
In two or more complete sentences define stratigraphy.:
Stratigraphy is a branch of geology concerned with the study of rock layers (strata) and layering (stratification). It is primarily used in the study of sedimentary and layered volcanic rocks.
In two or more complete sentences, explain the principle of superposition:
In physics and systems theory, the superposition principle, also known as superposition property, states that, for all linear systems, the net response at a given place and time caused by two or more stimuli is the sum of the responses which would have been caused by each stimulus individually
Define and compare the relationship between the following principles. Provide a supporting example for each comparison.
Original Horizontality
Original Lateral Extension
Cross-Cutting Relationships
Inclusion
Uniformities:
Original Horizontality- This principle stated by Nicholas Steno says that the gravitational pull deposits the sediment layers horizontally.
Original Lateral Extension- It states that a rock keeps on extending laterally unless a structural change prevents it.
Cross- cutting Relationships- This relationship states that if one geologic feature cuts another feature then it is younger of the two.
Inclusion- The inclusions observed in the sedimentary rocks if are in a formation then it concludes that the foundation is younger than the inclusion.
Uniformity- It states that all the geological processes are executed in a similar manner and a same intensity can be found in the past and in the present. This uniformity in the geological processes is enough for all the changes in geology.
The first two principles conclude that Earth was in the continuous process of formation and it still is because the deposit of layers and their constant extension and movements are the reason.
The inclusion and cross cutting are related as they both states about the sediment rock formations and the how it decides the ages of rock through changes. Uniformity is the bond that controls all the principles.
Your experiences and knowledge of the world are combined to create reality, which is how you perceive the world. and assess the world's condition based on reality rather than your desires.
What is Reality?No school or university teaches about objective reality. As a result, the majority of individuals unknowingly inhabit a fantasy universe.
Real is defined as having the capacity to be treated as fact, as existing or occurring in reality, as having a confirmed existence, and as having a nature that is consistent with reality.
Real is defined as something that is not an illusion, a fantasy, an imaginary world, or an intuitive emotion.
Therefore, Your experiences and knowledge of the world are combined to create reality, which is how you perceive the world. and assess the world's condition based on reality rather than your desires.
To learn more about Reality, refer to the link:
https://brainly.com/question/28607942
#SPJ2
Decide whether these proposed lewis structures are reasonable
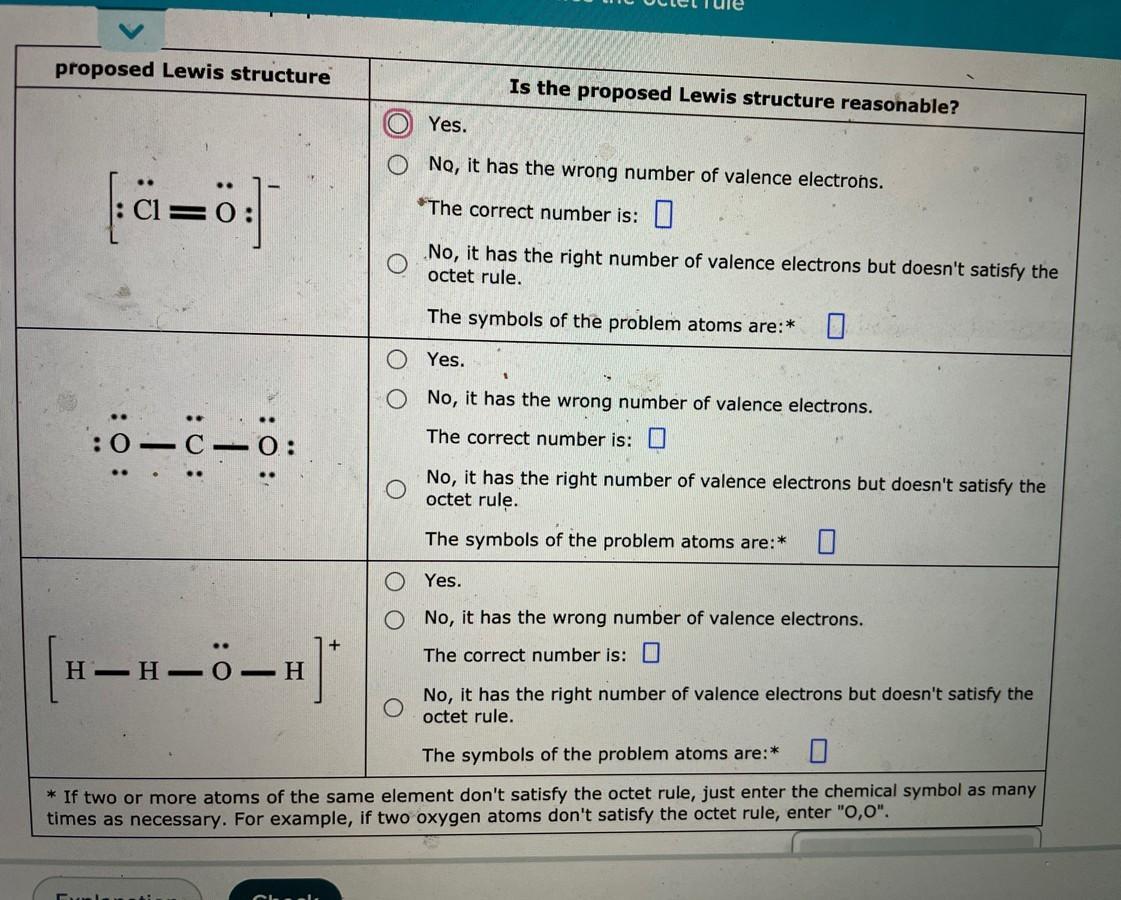
Answers
1) Yes, it is a reasonable Lewis structure
2) Yes, it is a reasonable Lewis structure
3) No, it has the wrong number of valence electrons
How do you know a reasonable Lewis structure?
A reasonable Lewis structure is determined by following a set of guidelines and rules, including:
Count the total number of valence electrons for all atoms in the molecule or ion.
Determine the central atom(s) in the molecule or ion based on its position in the periodic table and its ability to make multiple bonds.
Use single bonds to connect each outer atom to the central atom(s).
Distribute the remaining electrons in pairs around the outer atoms to complete their octets (or duets for hydrogen).
Place any remaining electrons on the central atom(s) to complete their octets, or use multiple bonds to do so.
Learn more about Lewis structure:https://brainly.com/question/20300458
#SPJ1
Among the following options, a valid Lewis structure of __________ cannot be drawn without violating the octet rule.
a. NH3
b. SbCl3
c. PF3
d. IF3
Answers
Answer:
d. IF3
Explanation:
The Octet rule posits that atoms gain, atom lose, or share electrons in order to have a full valence shell of 8 electrons. This statement occurs when atoms also combine to form molecules until they attain or share eight valence electrons either by losing or gaining eletrons.
From the given options, a valid Lewis structure that cannot be drawn without violating the octet rule is IF3
Question attached thank you

Answers
First, we shall determine mole of each gas present. This is shown below:
For argon, Ar
Mass of argon, Ar = 3.03 grams Molar mass of argon, Ar = 40 g/mol Mole of argon, Ar =?Mole = mass / molar mass
Mole of argon, Ar = 3.03 / 40
Mole of argon, Ar = 0.076 mole
For krypton, Kr
Mass of krypton, Kr = 1.16 grams Molar mass of krypton, Kr = 83.8 g/mol Mole of krypton, Kr =?Mole = mass / molar mass
Mole of krypton, Kr = 1.16 / 83.8
Mole of krypton, Kr = 0.014 mole
Finally, we shall determine the total pressure of the mixture. Details below:
Mole of argon, Ar = 0.076 mole Mole of krypton, Kr = 0.014 mole Total mole (n) = 0.076 + 0.014 = 0.09 moleVolume (V) = 2.50 LTemperature (T) = 25 °C = 25 + 273 = 298 KGas constant (R) = 0.0821 atm.L/mol KTotal pressure (P) =?PV = nRT
P × 2.5 = 0.09 × 0.0821 × 298
Divide both sides by 2.5
P = (0.09 × 0.0821 × 298) / 2.5
Total pressure = 0.88 atm
How do i determine the partial pressure?The partial pressure for each gas can be obtain as follow:
For argon, Ar
Mole of argon, Ar = 0.076 moleTotal mole = 0.09 moleTotal pressure = 0.88 atmPartial pressure Ar =?Partial pressure = (Mole / total mole) × total pressure
Partial pressure Ar = (0.076 / 0.09) × 0.88
Partial pressure Ar = 0.74 atm
For krypton, Kr
Partial pressure Ar = 0.74 atmTotal pressure = 0.88 atmPartial pressure of Kr =?Partial pressure of Kr = Total pressure - Partial pressure of Ar
Partial pressure of Kr = 0.88 - 0.74
Partial pressure of Kr = 0.14 atm
Learn more about partial pressure:
https://brainly.com/question/15577259
#SPJ1
Lexi is balancing equations. She is finding one equation to be very difficult to balance. Which explains how to balance the equation ZnSO4 + Li2CO3 → ZnCO3 + Li2SO4?
Answers
Answer:
deez
Explanation:
thx
Answer:
Atoms in the equation are already in balance
Explanation:
It's D
If you have 10,000 grams of a substance that decays with a half-life of 14 days, then how much will you have after 56 days? Round to the nearest whole number.
Answers
Answer:
2,500
Explanation:
I did mathematics and I'm smart as well
Answer:
Other answer is wrong. They are solving for this question.
"If you have 10,000 grams of a substance that decays with a half-life of 14 days, then how much will you have after 28 days?
Round to the nearest whole number"
The answer to the one asked above is 625. See math below.
Explanation:

how do one get this solution
-log10 (2* 10^-2)
Answers
The result of the computation when you follow the steps is 1.699.
A logarithm is a mathematical function that represents the exponent or power to which a specific base must be raised to obtain a given number. In simpler terms, it answers the question: "To what power must we raise a base number to obtain a certain value?"
What you should do is that on your calculator, you could press the logarithm key and then put in the value that has been shown and then the result would be displayed on your calculator.
Learn more about logarithm:https://brainly.com/question/30226560
#SPJ1
How many elements are present in the compound RbMnO4?
Answers
Rubidium
Manganese
Oxygen
Hope this helped☺️
sulfanomids warnings
Answers
Answer:
Sulfanomides Warnings
Sulfonamides may cause blood problems, especially if they're taken for a long period of time. These medicines can also cause a serious, even life-threatening, skin rash. Tell your doctor right away if you notice a rash or unusual skin changes.
Explanation:
Hope this helps you !!0/1 point
Click on the chemical compound that is a product in
this balanced chemical equation.
N+3H, → ẢNH,
Answers
NH3 is the chemical compound produced as a product in the given balanced chemical equation.
What is NH3 defined in chemistry?With the formula NH3, ammonia is a nitrogen and hydrogen inorganic chemical. A Lewis base is urea. Ammonia is a colourless, extremely unpleasant gas that has a strong, suffocating stink when it is present at room temperature. It is hygroscopic and known as anhydrous ammonia in its pure state (readily absorbs moisture). Ammonia is corrosive and has alkaline qualities.
Do NH3 and H2O have such a dipole moment?Water molecules' shared dipoles and development of hydrogen bonds with one another account for this. As a consequence, the molecules of water and ammonia are both made up of dipoles that exert significant electrostatic forces on one another.
Learn more about chemical equation here:
brainly.com/question/14072552
#SPJ1
Question 10 of 35
The graph shows the change in temperature of a sample
of water in a closed system as thermal energy is added
over time.
Temperature (°C)
150°C
100°C.
50°C-
g
0°C-
-50°C
10
20 30 40 50
Time (min)
What happens to the temperature of the water when it begins to melt?
OA The temperature remains at 100°C until the change of state is
complete
B. The temperature continues to increase during the change of state
C. The temperature continues to decrease during the change of
state.
OD. The temperature remains at 0°C until the change of state is
complete.

Answers
Answer:
D. The temperature remains at 0°C until the change of state is complete.
which 2 characteristics must and object have in order to be considered matter?
Answers
Answer:.
Explanation: Matter must have Mass and Volume to be considered matter!
• How does the name of the salt tell us that:
a) there is just one other element combined with the metal?
b) there is oxygen present in the salt?
Answers
The name of the salt tells us that:
a) there is just one other element combined with the metal by looking at the suffix of the salt's name.
b) the presence of oxygen in a salt can be indicated by the name of the salt.
a) The name of a salt can tell us that there is just one other element combined with the metal by looking at the suffix of the salt's name. If the salt name ends in "-ide," it indicates that the salt is composed of a metal and a single non-metal element.
For example, sodium chloride (NaCl) and potassium bromide (KBr) are salts where the metal (sodium and potassium) is combined with a single non-metal element (chlorine and bromine, respectively). The "-ide" suffix suggests the presence of only one other element in the salt.
b) The presence of oxygen in a salt can be indicated by the name of the salt. If the salt name includes the element oxygen, it suggests that oxygen is present in the salt compound.
For example, sodium carbonate (Na₂CO₃) and calcium sulfate (CaSO₄) contain the element oxygen in their chemical formulas. The presence of oxygen in the salt is implied by the name and the combination of elements in the compound.
Therefore, the name of salt tells us that there is just one other element combined with the metal and there is oxygen present in the salt
Learn more about salt here:
https://brainly.com/question/31814919
#SPJ 1
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Scientists study and record seismic data and volcanic activity in order to support: Convection in the Lithosphere Theory of Plate Tectonics Sea Floor Spreading Theory of Continental Drift
Answers
Answer:
Theory of Plate Tectonics
Explanation:
Scientists especially Geologists study and record seismic data and volcanic activities in order to support the theory of plate tectonics.
The theory of plate tectonics suggests that the earth is broken into slabs called the lithosphere that moves over the fluid asthenosphere below.
The use of seismic data presents scientists with a way of vertically looking deep into the earth. Volcanic activities also presents us with the kind of materials down within the earth. Also, coupled with this data, scientists use GPS sensors to monitor the rate of movement of the earth. When studying plate tectonics, it invariably culminates in the study of sea floor spreading and theory of continental drift.10. For the reaction
H₂(g) + O₂(g) → H₂O(l)
H=-286 kJ/mol
What is the enthalpy change when 10.4 mol of hydrogen gas reacts with excess oxygen?
a. 27.5 kJ
b.-27.5 kJ
c. 3.64 x 10-2 J
d. -2.97 × 10³ J
e. -1.48 x 10³ J