Answers
Answer:
Because it is!
Explanation:
Hurricanes was the one of the big natural disaster . Yes, it was true and it is in the book of mythology.
What made hurricanes Katrina so powerful ?It was so destructive primarily because levees around New Orleans, Louisiana failed. Levees are water barriers built to prevent flooding (parts of New Orleans have an elevation that is lower than sea level). When the levees failed, huge areas of the cities flooded.
Water is in constant motion, moving from the ocean to the sky to the earth. Flooding is a natural event which occurs when water overflows a stream, river or drain. Floods are caused by rainfall, melting snow or ice, or by the failure of structures such as dams.
There are main four parts of water cycle . i.e. evaporation , convection , precipitation and - collection.
To learn more about hurricanes Katrina, follow the link below:
https://brainly.com/question/1445023
#SPJ2
Related Questions
Which two atoms are isotopes of each other? A. Si with a mass number of 27 and an atomic number 14, and Mg with a mass number of 25 and an atomic number of 12. B. Mg with a mass number of 24 and an atomic number 12, and Al with a mass number 24 and an atomic number 13. C. Mg with a mass number 24 and an atomic number 12, Mg with a mass number 25 and an atomic number 12. D. Na with a mass number 23 and an atomic number 11, Mg with a mass number 25 and an atomic number 12.
Answers
The two atoms are isotopes of each other are Si with a mass number of 27 and an atomic number 14, and Mg with a mass number of 25 and an atomic number of 12. The correct option is A.
What are isotopes?Isotopes are atoms with the same number of protons but differing numbers of neutrons.
They differ in mass, which affects their physical characteristics even if they have nearly identical chemical properties.
An isotope is one of two or more chemical elements that exist in different forms. Varied isotopes of an element share the same atomic number and protons in their nuclei, giving them the same atomic weight.
However, each elemental isotope has a different amount of neutrons, which changes its atomic weight.
They can be referred to as isotopes because Si, which has a mass number of 27 and an atomic number of 14, and Mg, which has a mass number of 25 and an atomic number of 12, both have 13 protons.
Thus, the correct option is A.
For more details regarding isotopes, visit:
https://brainly.com/question/11680817
#SPJ1
lewis structure of HONO2 (HNO3)
Answers
I really hope this diagram helped you.
If you have further questions don't hesitate to ask :)

What's the molality of a solution with 120 g of NaCl and 30 kg of water?
Answers
Answer:
0.07mol/kg
Explanation:
Given parameters:
Mass of NaCl solution = 120g
Mass of water = 30kg
Unknown:
Molality of the solution =?
Solution:
The molality of a solution is the number of moles of the solute per mass of the solution.
Molality = \(\frac{number of moles of solute}{mass of solution}\)
Number of moles of NaCl = \(\frac{mass}{molar mass}\)
Molar mass of NaCl = 23 + 35.5 = 58.5g/mol
Number of moles of NaCl = \(\frac{120}{58.5}\) = 2.05mole
So;
molality = \(\frac{2.05}{30}\) = 0.07mol/kg
Answer:
0.068
Explanation:
Which intermolecular force(s) do the following pairs of molecules experience? (Consider asking yourself which molecule in each pair is dominant?)

Answers
The Fischer projection given here is for L-enantiomer and ketopentose. Option B is correct, as there is a ketone group present in the carbohydrate monomer. Carbohydrate can have an aldehyde or ketone as a functional group.
What is a carbohydrate?
Carbohydrate monomer is a single unit that can either have an aldehyde functional group called aldose or a ketone functional group called ketose. Here in the given diagram, the monomer has a ketone group (C=O) and has five carbons, so it is considered a keto pentose. Carbohydrates are classified into L and D forms based on their OH group, and this L and D form differs from the "l" and "d" form (classified on the direction of movement around the plane polarized light).
Hence, this given diagram is L-enantiomer and keto pentose, which is option B.
Learn more about carbohydrates here.
https://brainly.com/question/13265192
#SPJ1
Consider the following reaction mechanism and its associated energy diagram:
S1: C₂H4 (g) + HBr(g) --> C₂H5+ + Br
S2:
C₂H5 + Br --> C₂H5Br (1)
Answers
Answer:
From the energy diagram, we can see that the reaction mechanism is an example of a two-step reaction. The first step (S1) involves the combination of ethylene (C₂H4) and hydrogen bromide (HBr) to form an ethyl cation (C₂H5+) and a bromide anion (Br). In the second step (S2), the ethyl cation and bromide anion combine to form ethyl bromide (C₂H5Br). The reaction is exergonic as evidenced by the fact that the activation energy, represented by the peak in the energy diagram, is negative. This indicates that the reaction releases energy.
The precise mass of 85X is 84.912 amu, and 72.17% of all atoms have that mass. The precise mass of 87X is 86.909 amu, and 27.83% of all atoms have that mass. Show the calculation to determine the average atomic mass for element X. Include a label on your answer.
Answers
The average atomic mass of element X, given the data is 85.469 amu
How to determine the avaerage atomic mass
Let 85X be isotope ALet 87X be isotope BFrom the question above, the following data were obtained:
Mass of isotope A (85X) = 84.912 amuAbundance of A (A%) = 72.17%Mass of isotope B (87X) = 86.909 amuAbundance of B (B%) = 27.83%Average atomic mass =?The average atomic mass of X can be obtained as illustrated below:
Average atomic mass = [(Mass of A × A%) / 100] + [(Mass of B × B%) / 100]
Average atomic mass = [(84.912 × 72.17) / 100] + [(86.909 × 27.83) / 100]
Average atomic mass = 61.282 + 24.187
Average atomic mass = 85.469 amu
Thus, the average atomic mass is 85.469 amu
Learn more about isotope:
https://brainly.com/question/14041912
#SPJ1
The solubility product constant of calcium sulfate, CaSO4, is 7.10 x 10-5. Its molar
mass is 136.1 g/mol. How many grams of calcium sulfate can dissolve in 75.0 L of
pure water?
Answers
Therefore, 86.1 grams of calcium sulfate can dissolve in 75.0 L of pure water at equilibrium.
What is the calcium sulfate equilibrium constant?Only a small amount of calcium sulphate is available. The equilibrium constant, also referred to as the solubility product, is written as Ks for this kind of dissolution process. Ks = 4.9 10 6 for this reaction. K s = 4.9 10 6.
The formula for calcium sulphate's solubility product constant is:
Ksp = [Ca2+][SO42-]
The solubility product constant, Ksp, is equivalent to the product of the molar concentrations of Ca2+ and SO42- at equilibrium:
[Ca2+][SO42-] = Ksp
The following calculation can be used to determine the molar solubility of calcium sulphate:
Ksp = [Ca2+][SO42-] = x²
where x is the molar solubility of calcium sulfate.
Therefore, x = √(Ksp) = √(7.10 x 10⁻⁵) = 8.43 x 10³M
m = n × M
n = V × C
Substituting the given values, we get:
n = 75.0 L × 8.43 x 10⁻³ mol/L = 0.632 mol
m = 0.632 mol × 136.1 g/mol = 86.1 g
To know more about calcium sulfate visit:-
https://brainly.com/question/7962933
#SPJ9
How many moles of
Cts are needed to make
15.5 moles of CO₂? How
much O2 will be needed?
0₂
C₂Hs +50₂3CO₂ + 4H₂O
Answers
1. The number of mole of C₃H₈ needed to make 15.5 moles of CO₂ is 5.2 moles
2. The number of moles of O₂ needed is 25.8 moles
1. How do I determine the number of mole of C₃H₈ needed?The number of mole of C₃H₈ needed can be obtained as illustrasted below:
C₃H₈ + 5O₂ -> 3CO₂ + 4H₂O
From the balanced equation above,
3 moles of CO₂ was obtained from 1 mole of C₃H₈
Therefore,
15.5 moles of CO₂ will be obtain from = (15.5 × 1) / 3 = 5.2 moles of C₃H₈
Thus, number of mole of C₃H₈ needed is 5.2 moles
2. How do I determine the number of mole of O₂ needed?We can obtain the number of mole of O₂ needed as follow:
C₃H₈ + 5O₂ -> 3CO₂ + 4H₂O
From the balanced equation above,
3 moles of CO₂ was obtained from 5 moles of O₂
Therefore,
15.5 moles of CO₂ will be obtain from = (15.5 × 5) / 3 = 25.8 moles of O₂
Thus, number of mole of O₂ needed is 25.8 moles
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
Complete question:
How many moles of C₃H₈ are needed to make
15.5 moles of CO₂? How much O₂ will be needed?
C₃H₈ + 5O₂ -> 3CO₂ + 4H₂O
Help please! I'll award brainliest and extra points if you show your work too :)

Answers
a. This reaction is endothermic because energy is absorbed during the reaction.
b. ΔH = +187 kJ
What is the energy change of the reaction?c. The balanced chemical equation for the decomposition of calcium carbonate is:
CaCO3(s) → CO2(g) + CaO(s)
d. We can use the molar mass of calcium carbonate to convert 21.0 g of CaCO3 to moles:
21.0 g CaCO3 x (1 mol CaCO3 / 100.09 g CaCO3) = 0.210 mol CaCO3
From the balanced equation, we can see that the molar ratio of CaCO3 to ΔH is 1:1. Therefore, the energy absorbed can be calculated as:
0.210 mol CaCO3 x 187 kJ/mol = 39.3 kJ
Therefore, 39.3 kJ of energy is absorbed during the decomposition of 21.0 g of calcium carbonate.
Learn more about energy changes of reactions at: https://brainly.com/question/21357822
#SPJ1
If you take several measurements, then your data will be precise if
Answers
If you take several measurements, then your data will be precise if the measurements are consistently close to each other.
What is precision?Precision is a measure of the reproducibility of a set of measurements. If a set of measurements is precise, it means that the measurements are similar to each other and give a reliable indication of the true value of the quantity being measured. On the other hand, if the measurements are widely spread out and inconsistent, they are considered imprecise.
Thus, to ensure precision in measurements, it's important to use accurate instruments, take multiple measurements, and use statistical methods to analyze the data and identify any outliers or errors. It's also important to follow standard procedures and protocols for making measurements to minimize any sources of variability.
Learn more about precise here: https://brainly.com/question/1578168
#SPJ1
Look at the diagram of a fuel cell below.
Which part of the fuel cell does A represent?
air
anode
cathode
electrolyte
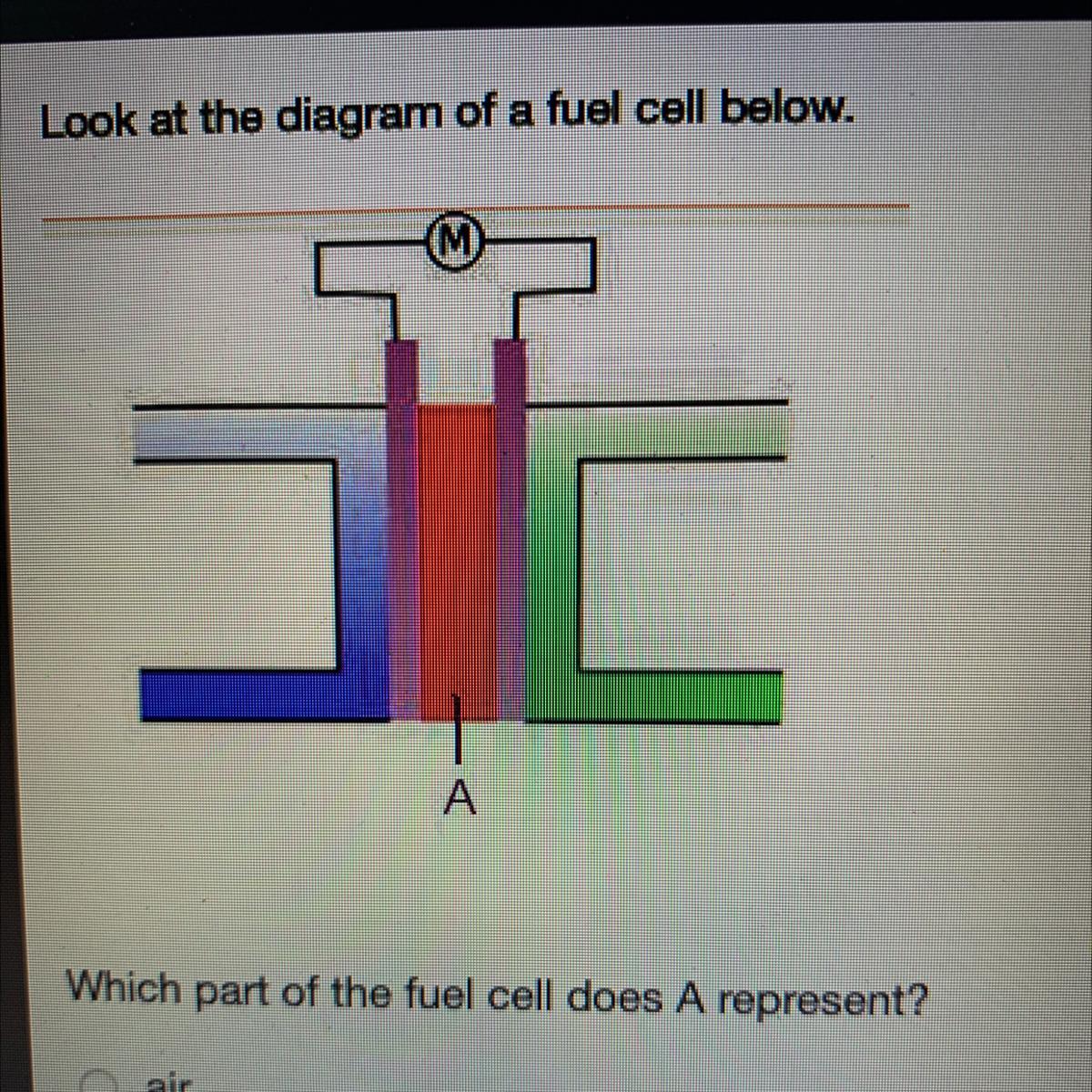
Answers
Answer:
I think its d) electrolyte
Explanation:
In the given diagram, The part of the fuel cell A represent is electrolyte.
What is Fuel Cell ?A fuel cell is the combination of two electrodes—anode (a negative electrode) and a cathode (positive electrode)— which is available around an electrolyte.
A hydrogen gas, is inserted to the anode, and air is inserted to the cathode.
Therefore, In the given diagram, The part of the fuel cell A represent is electrolyte.
Learn more about Fuel cell here ;
https://brainly.com/question/4607420
#SPJ5
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Liquid octane (CH3(CH2)6CH3) will react with gaseous oxygen (O2) to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). Suppose 17. g of octane is mixed with 112. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.
Answers
The maximum mass of water that could be produced by the chemical reaction is 162 g.
The given chemical equation is: 2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(l)In the chemical reaction of liquid octane with gaseous oxygen, the products are gaseous carbon dioxide and gaseous water.According to the balanced chemical equation, 2 moles of C8H18 react with 25 moles of O2 to form 18 moles of H2O.So, 1 mole of C8H18 react with 25/2 = 12.5 moles of O2 to form 9 moles of H2O.The molar mass of C8H18 is 114 g/mol. So, the number of moles in 17 g of C8H18 is:17 g / 114 g/mol = 0.149 molThe molar mass of O2 is 32 g/mol. So, the number of moles in 112 g of O2 is:112 g / 32 g/mol = 3.5 molFrom the balanced chemical equation, 1 mole of C8H18 react with 12.5 moles of O2 to form 9 moles of H2O.So, the number of moles of O2 required to react with 0.149 mol of C8H18 to form H2O is:(12.5 mol / 1 mol) × (0.149 mol / 2 mol) = 0.935 molThe maximum number of moles of H2O that can be produced from 0.149 mol of C8H18 and 0.935 mol of O2 is 9 mol.So, the mass of water produced from 17 g of C8H18 and 112 g of O2 is:9 mol × 18 g/mol = 162 g
for more questions on chemical reaction
https://brainly.com/question/25769000
#SPJ8
2Fe2O3 + 3C → 4Fe + CO2
IS this a balanced equation?
Answers
Answer:
No
Explanation:
In the left side of the reaction, there are 6O and 3C but on the right side, there are only 1C and 2O
what are the properties of hot sodium? what are the properties of chlorine gas?
Answers
Answer:
soft metal, reactive and with a low melting point, with a relative density of 0,97 at 20ºC (68ºF) for chlorine liquid and solid form it is a powerful oxidizing, bleaching, and disinfecting agent
Explanation:
Suppose a student repeats Experiment 1 using strontium instead of magnesium. The student adds 4.93 g of strontium to a crucible, heats the crucible and its contents for several minutes over a Bunsen burner, and records the final mass of the crucible and its contents.
Write the balanced chemical equation for this reaction. Include physical states.
balanced equation:
What mass of product is expected to form in this reaction? Assume all of the strontium reacts.
mass of product:
Answers
The balanced chemical equation for the reaction between strontium and oxygen can be written as follows: 2 Sr (s) + \(O_2\)(g) → 2 SrO (s).
In this equation, solid strontium (Sr) reacts with gaseous oxygen (\(O_2\)) to produce solid strontium oxide (SrO).
To determine the mass of product expected to form in this reaction, we need to consider the molar ratio between strontium and strontium oxide. From the balanced equation, we can see that 2 moles of strontium react to produce 2 moles of strontium oxide.
The molar mass of strontium (Sr) is 87.62 g/mol, and the molar mass of strontium oxide (SrO) is 119.62 g/mol. Since the molar ratio is 1:1 between strontium and strontium oxide, the mass of strontium oxide formed will be equal to the mass of strontium used.
In this case, the student added 4.93 g of strontium to the crucible. Therefore, the expected mass of strontium oxide formed will also be 4.93 g.
It's important to note that this calculation assumes that the reaction proceeds to completion, meaning that all of the strontium reacts with oxygen. In actual laboratory conditions, the yield of the reaction may be less than 100% due to factors such as incomplete reaction, side reactions, or product loss.
For more such questsion on balanced chemical equation visit:
https://brainly.com/question/11904811
#SPJ8
A person is told to take 1.25 mg of vitamin D daily. How many 0.250 mg tablets should this person take
Answers
5 tablets must be taken by the patient each day.
In a randomized clinical experiment with nursing home residents, the effects of equal oral vitamin D3 doses of 600 IU/day, 4200 IU/week, and 18,000 IU/month on vitamin D status were compared. The most efficient dosage was a daily dose, followed by a weekly dose and a monthly dose. It is believed that vitamin D status is affected equally by equivalent daily, weekly, or monthly doses of vitamin D3. A randomized clinical experiment including nursing home residents looked at this.
we know that for 1 day we need to divide 1.25 by 0.250
so, the answer will be 5 pills per day.
Learn more about Vitamin D here-
https://brainly.com/question/15080220
#SPJ9
Joaquin tells his science class that galaxies consist of gas, dust, and many planets. What is the most important component of galaxios Joaquin is missing in his description?
astero de
comets
constellations
Stars
Answers
Answer:
the answer is asteroids!
Explanation:
Answer:
astroid
Explanation:
becaus
The specific heat of water is 4.184 J/gºC. How much energy (in
joules) is required to raise the temperature of 18g of H2O from
283K to 293K?
Answers
mark brainleist
Solar and wind energy are both intermittent resources that cannot be relied upon for a constant stream of energy production. Explain why developing better ways to store energy is an important part of making these energy sources more practical to use.
Answers
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers.
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers. Energy storage's inherent ability to offer backup power in the event of grid failure is a feature that both residential consumers and commercial owners find highly desirable.
To know more about energy, here:
https://brainly.com/question/1932868
#SPJ1
Question 10 of 10
Choose the option which best answers the
question.
Which of the following is not equivalent to
one drink?
a. 12 oz. of beer
b. 5 oz. of wine
c. 4 oz. of 40-proof liquor
d. 1 1/2 oz. of 80-proof liquor
Answers
Answer:
d 1 1/2 oz.. of 80-proof liquor
1 1/2 oz. of 80-proof liquor is not equivalent to one drink. Hence, option D is correct.
What is oz?Oz is an abbreviation for ounce. An ounce is equal to 1/16 pound (437 1/2 grains) and equal to 480 grains, or 1/12 pound.
1 1/2 oz. of 80-proof liquor is not equivalent to one drink.
Hence, option D is correct.
Learn more about oz here:
https://brainly.com/question/1396131
#SPJ5
1) To increase the amount of NH3 at 200 atm, the manufacturer should (increase, decrease, not change) the temperature of the reaction chamber.
2) This change in temperature would shift the reaction to the (left, right) because this equilibrium reaction is (exothermic, endothermic)
Answers
The temperature of the reaction should be decreased
This change in temperature would make the equilibrium to shift to the right.
What is the LeChatelier principle?
The Le Chatelier's principle, commonly referred to as the Le Chatelier's principle of equilibrium, is a chemical principle that describes how an equilibrium system reacts to environmental changes.
According to this theory, when an equilibrium system is exposed to an outside force, it will respond in a way that partially offsets the imposed change and restore equilibrium.
Learn more about LeChatelier principle:https://brainly.com/question/31377984
#SPJ1
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
Since the lattice structure of sodium chloride consists of thousands of sodium ions and chloride ions attracted to each other, the lattice structure of sodium chloride does not dissolve in water.statement: True or False
Answers
Answer: the statement presented by the question ("the lattice structure of sodium chloride does not dissolve in water") is false.
Explanation:
The question requires us to determine if the following statement is true or false: "the lattice structure of sodium chloride does not dissolve in water".
Sodium chloride (NaCl) is an ionic compound, which means that the atoms of sodium (Na) and chlorine (Cl) are attatched together through electrostatic interaction between their respective ions (Na+ and Cl-).
Since water is a polar molecule, the positive parts of water molecule are able to interact with the anions from an ionic compound (such as Cl- in NaCl), while the negative parts of water molecule are able to interact with the cations from an ionic compound (such as Na+ in NaCl). This way, water as a solvent is capable of dissolving NaCl - because of the interaction between the polar molecule and the ions in the ionic compound.
Therefore, we can say that the lattice structure of sodium chloride does dissolve in water, and the statement presented by the question ("the lattice structure of sodium chloride does not dissolve in water") is false.
Which of the following is a true statement?
All flat-topped hills are plateaus.
Volcanoes form all flat-topped hills.
All flat-topped hills are mesas.
Flat-topped hills can either be plateaus or mesas.
Answers
Answer:
D Flat Top Hills Can Be Plateaus or mesas
Explanation:
Which of the liquids you tested (isopropyl alcohol, water, and glycerol) displayed the greatest surface tension (greatest intermolecular forces)?
Answers
to be my friend and I think
What is the density at 27 °C of 28.0 milliliters of a liquid that has a mass of 4.05 grams?
Answers
Answer:
The answer is 0.14 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass = 4.05 g
volume = 28 mL
We have
\(density = \frac{4.05}{28} \\ = 0.1446428571...\)
We have the final answer as
0.14 g/mLHope this helps you
Why are chemical equilibria called dynamic equilibria?
Answers
Answer:
Because molecules are moving so they change from reactants to products and vice versa.
Explanation:
Hello,
In this case, it is widely known that chemical equilibrium is related with such condition at which the rate of consumption of reactants and formation of products tends to zero so their concentrations remain the same over the time. For instance, if we consider the formation of hydrogen chloride at equilibrium:
\(H_2+Cl_2\rightleftharpoons 2HCl\)
We can also see that the reactants, hydrogen and chlorine are consumed to form hydrogen chloride and it also goes back to its reactants in a dynamic equilibrium (because they are constantly changing).
Best regards.
What are the lengths of the diagonals of the kite?

Answers
The answer ( 13 and 8 )
x²=5²+12²
x²=25+144
x²=169
x=13
x²=5²+6²
x²=25+36
x²=61
x=7.8
x=8
If the vapor pressure of water at 20.0 o C is 17.535 torr, and the atmospheric pressure measured by a barometer was 757.3 torr, what is the partial pressure of H2 in the gas mixture in a buret
Answers
Answer:
f the vapor pressure of water at 20.0 o C is 17.535 torr, and the atmospheric pressure measured by a barometer was 757.3 torr, what is the partial pressure of H2 in the gas mixture
Partial pressure of H2 in gas mixture is :
739.765 torr .
Explain total pressure?The overall pressure ptot is the total of all pressures in a reference system. This pressure, according to Bernoulli (see Fluid mechanics), consists of the static pressure p, the dynamic pressure pdyn, and the geodetic component ( g z) that exists in a fluid along a stream line in a frictionless flow.The total pressure of a gas mixture is the sum of its component gases' partial pressures: Ptot = ∑Pi = P1 + P2 + P3... ntot = total number of moles in the gas mixture (the sum of all ni).Total pressure of mixture =
Partial of hydrogen + Partial pressure of oxygen
Partial of Hydrogen = Total pressure - partial pressure of oxygen
Here total pressure = 757.3 torr
Partial pressure of oxygen = 17 . 535 torr
Total pressure = 757 . 3 - 17 . 533
= 739.765 torr.
To learn more about total pressure refer to :
https://brainly.com/question/22264785
#SPJ2