Answers
Answer:
Mass = 100.8 g
Explanation:
Given data:
Mass of sulfur formed = ?
Mass of water formed = 37.4 g
Solution:
Chemical equation:
2H₂S + SO₂ → 3S + 2H₂O
Number of moles of water:
Number of moles = mass/molar mass
Number of moles = 37.4 g/ 18 g/mol
Number of moles = 2.1 mol
Now we will compare the moles of water and sulfur.
H₂O : S
2 : 3
2.1 : 3/2×2.1 = 3.15
Mass of sulfur:
Mass = number of moles × molar mass
Mass = 3.15 mol × 32 g/mol
Mass = 100.8 g
Related Questions
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
How do I go about solving a Nuclear Equation?

Answers
Answer:
How do amoeba respire.
How do plants respire.
What is the molarity of a solution that contains 0.75 mol Naci in 3.0 L of solution? Select one: O a. 4.0 M O b. 2.3 M O d. 3.8 M O d. 0.25 M Clear my choice
Answers
Answer:
\(\boxed {\boxed {\sf D. \ 0.25 \ M}}\)
Explanation:
Molarity is a measure of concentration in moles per liter.
\(molarity= \frac{moles \ of \ solute}{ liters \ of \ solution}\)
The solution contains 0.75 moles of sodium chloride and has a volume of 3.0 liters.
moles of solute = 0.75 mol NaCl liters of solution = 3.0 LSubstitute these values into the formula.
\(molarity= \frac{ 0.75 \ mol \ NaCl}{3.0 \ L}\)
\(molarity= 0.25 \ mol \ NaCl/L\)
Molarity has the molar (M) as its unit. 1 molar is equal to 1 mole per liter.
\(molarity= 0.25 \ M \\)
The molarity of the solution is 0.25 Molar and Choice D is correct.
Is calcium oxide a Element or compound or mixture?
Answers
Answer:
compound
Explanation:
Answer:
The answer is compound
Explanation:
A muffi n recipe calls for cream of tartar, or potassium
hydrogen tartrate, KHC4H4O6(s). Th e amount of
cream of tartar that is required contains 2.56 × 1023
atoms of carbon. What amount in moles of
potassium hydrogen tartrate is required?
Answers
A muffi n recipe calls for cream of tartar, or potassium hydrogen tartrate. The amount of cream of tartar that is required contains 2.56 ×10²³atoms of carbon. 0.42moles of potassium hydrogen tartrate is required
In the Global System of Units (SI), the mole represents the unit of material quantity. How many fundamental entities of a particular substance are present within an object a sample is determined by the quantity of that material. An elementary entity can be a single atom, a molecular structure, an ion, a charged particle pair, or a particle that is subatomic like a proton depending on the makeup of the substance.
For instance, despite the fact that the two substances have different volumes and masses, 10 moles of water because 10 moles of the chemical element mercury both contain the same quantity of stuff, because the mercury comprises exactly one particle for each molecule of water.
mole = 2.56 ×10²³/ 6.022×10²³
= 0.42moles
To know more about mole, here:
https://brainly.com/question/26416088
#SPJ1
How many atoms are in 12 g of Carbon-12 (12C)?
Answers
There are approximately 6.022 × 10^23 atoms in 12 grams of Carbon-12 (12C).
The number of atoms in a given amount of a substance can be calculated using Avogadro's number, which represents the number of atoms or molecules in one mole of a substance. Avogadro's number is approximately 6.022 × 10^23.
Carbon-12 is a specific isotope of carbon, with an atomic mass of 12 atomic mass units (amu). One mole of Carbon-12 has a mass of 12 grams. Since one mole of any substance contains Avogadro's number of particles, in the case of Carbon-12, it contains 6.022 × 10^23 atoms.
Therefore, if we have 12 grams of Carbon-12, which is equal to one mole, we can conclude that there are approximately 6.022 × 10^23 atoms in this amount of Carbon-12.
In summary, 12 grams of Carbon-12 contains approximately 6.022 × 10^23 atoms. Avogadro's number allows us to relate the mass of a substance to the number of atoms or molecules it contains, providing a fundamental concept in chemistry and enabling us to quantify and understand the microscopic world of atoms and molecules.
for such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
In the organization of living things, tissues combine to form
A)Cells
B)communities
C) organisms
D)Organs
Answers
Answer:
D
Explanation:
Answer:d
Explanation:
What is the zonecreated if force of separation occurs?
Answers
What forms of energy are produced when
fossil fuels burn?
Answers
When fossil fuels burn, several forms of energy are produced, including:
Heat energy: The primary form of energy released during fossil fuel combustion is heat. Fossil fuels contain chemical energy stored for millions of years, and when they burn, this energy is released in the form of heat. The heat energy can be harnessed for various purposes, such as heating buildings or generating steam to drive turbines.
Light energy: Burning fossil fuels can also produce light energy in the form of flames or glowing embers. This light energy is a byproduct of combustion.
Mechanical energy: Heat generated by burning fossil fuels can be converted into mechanical energy. This is typically achieved by using heat to produce steam, which drives a turbine connected to a generator. The rotating turbine converts the heat energy into mechanical energy, which is further transformed into electrical energy.
Electrical energy: Through the process described above, burning fossil fuels can ultimately generate electrical energy. The mechanical energy produced by the turbine is converted into electrical energy by the generator. Electrical energy can power various devices, appliances, industries, and infrastructure.
It's critical to note that while burning fossil fuels can produce useful forms of energy, it also results in the release of carbon dioxide and other greenhouse gases. This contributes to climate change and environmental concerns. As a result, there is a global shift towards cleaner and renewable energy sources to mitigate these negative impacts.
Systemic lupus erythematosus, or lupus, affects a few million people in the United States.
Most of these people are young women. Lupus is a chronic, autoimmune disease that
affects connective tissue in any part of the body. An attack can damage organs and the
nervous system. What conclusion can be drawn about lupus?
A. Most victims of lupus are middle-aged women.
B. Hormones most likely are unrelated to the lupus disease.
C. Lupus is easy for a physician to diagnose.
D. Lupus can affect almost every system of the body.
E. It is uncommon to have skin problems with lupus.

Answers
The conclusion that can be drawn about lupus is that: Lupus can affect almost every system of the body. Option D. is the correct answer.
What is Lupus (SLE)?
This refers to a disease that happens when your body's immune system attacks your own tissues and organs (autoimmune disease).
Lupus (SLE) can affect the following body parts: joints, skin, kidneys, blood cells, brain, heart and lungs.
Symptoms differ from person to person but can include fatigue, joint pain, rash and fever. These can get worse from time to time (flare up) and then improve.
Lupus has no cure. Current treatments are based on improving quality of life by controlling symptoms and minimizing flare-ups. This starts with lifestyle modifications, including sun protection and diet. Also, disease management includes medication such as anti-inflammatories and steroids.
Learn more about Lupus on
https://brainly.com/question/28445821
#SPJ1
What is the role of a decomposer in a food chain?
A. to move food from producers to consumers
B. to move food from consumers to other consumers
C. to make food for the ecosystem
D. to return matter to the environment
Answers
Answer:D
Explanation:
Decomposers decompose food and return it to the environment through the soil
Which of these accurately describes the photoelectric effect?
A. Shining a LOW frequency light over a metal will cause PROTONS to be ejected.
B. Shining a HIGH frequency light over a metal will cause ELECTRONS to be ejected.
C. Shining a LOW frequency light over a metal will cause ELECTRONS to be ejected.
D. Shining a HIGH frequency light over a metal will cause PROTONS to be ejected.
Answers
The statement that accurately describes the photoelectric effect is Shining a HIGH frequency light over a metal will cause ELECTRONS to be ejected. That is option B.
What is photoelectric effect?Photoelectric effect is defined as the process by which electrically charged particles are released from or within a material when it absorbs electromagnetic radiation.
The photoelectric effect can also be defined as the ejection of electrons from a metal plate when light falls on it.
Therefore, statement that accurately describes the photoelectric effect is Shining a HIGH frequency light over a metal will cause ELECTRONS to be ejected.
Learn more about electrons here:
https://brainly.com/question/25674345
#SPJ1
Which of these is a chemical property?
boiling point
odor
ability to rust
color
Answers
Answer:
ability to rust
Explanation:
i'm like 90% sure thats correct
Write the correct IUPAC name for the following compounds: Mg(NO3)2 ,CaCl2 S2Cl4 ,HI (aq) and FeBr3
Answers
IUPAC name is the systematic name given to a chemical compound according to the rules and guidelines set forth by IUPAC.
What does IUPAC stand for?IUPAC is short for International Union of Pure and Applied Chemistry. The IUPAC name provides a standard and unambiguous way of naming chemical compounds, which is important for communication among scientists and for the accurate reporting of chemical information.
The IUPAC name is typically based on the structure and composition of the compound, and it provides information about the functional groups and other features of the molecule. The naming rules take into account factors such as the number and type of atoms, the arrangement of atoms within the molecule, and the presence of functional groups.
The accurate IUPAC names for the given substances are:
Mg(NO3)2: Magnesium nitrate
CaCl2: Calcium chloride
S2Cl4: Disulfur tetrachloride
HI (aq): Hydrogen iodide or Hydroiodic acid
FeBr3: Iron(III) bromide
To find out more about IUPAC names, visit:
https://brainly.com/question/30086566
#SPJ1
Complete the equation for the conversion of sucrose into glucose
(1)C12H22O11 + H2O
Answers
Answer:
C₁₂H₂₂O₁₁ + H₂O → C₅H₁₂O₆ + C₆H₁₂O₆
Explanation:
Chemical equation:
C₁₂H₂₂O₁₁ + H₂O → C₅H₁₂O₆ + C₆H₁₂O₆
Source of sucrose:
Sucrose is present in roots of plants and also in fruits. It is storage form of energy. Some insects and bacteria use sucrose as main food. Best example is honeybee which collect sucrose and convert it into honey.
Monomers of sucrose and hydrolysis:
Sucrose consist of monomers glucose and fructose which are join together through glycosidic bond. Hydrolysis break the sucrose molecule into glucose and fructose. In hydrolysis glycosidic bond is break which convert the sucrose into glucose and fructose. Hydrolysis is slow process but this reaction is catalyze by enzyme. The enzyme invertase catalyze this reaction.
The given reaction also completely follow the law of conservation of mass. There are equal number of atoms of elements on both side of chemical equation thus mass remain conserved.
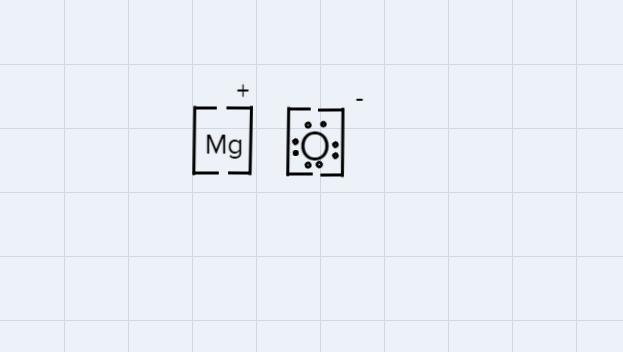
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?

Answers
Answer:
C.
Explanation:
The correct way to write the diagram of an ionic compound is:
- Use brackets by writing inside them, the symbol of each element separately.
- Draw the symbol and the number o the charge of each one, negative (-) add positive +()., in this case it is +1 for Mg (because Mg lost an electron) and -1 for O (because O won an elec).
Which of the following particles has the smallest
mass?
A) electron
C) neutron
B) hydrogen atom
D) proton
Answers
Answer:
AN ELECTRON HAS THE SMALLEST MASS!
Answer:
A) electron
good luck, i hope this helps :)
Is glue a liquid or a solid
Answers
Answer:
White school glue is liquid because its long polymers can slide over and along one another. It does not flow easily, though; it is quite viscous. The addition of some chemicals—such as a borax solution (or sodium tetraborate decahydrate dissolved in water)—can cause cross-links to form between the polymers.
Explanation:
Answer:
It is a liquid :]
Can H2 be broken down? (Not H)
Answers
Hello, this is Bing. I can help you with your question. Based on the information I found on the web, **H2** can be broken down into its two atoms of hydrogen (H) by supplying enough energy to overcome the bond that holds them together⁴. This process is called **dissociation** and requires an energy equal to or greater than the **dissociation energy** of H2, which is about 436 kJ/mol⁴.
One way to break down H2 is by using **electricity** to split water (H2O) into hydrogen (H2) and oxygen (O2) through a process called **electrolysis**¹. In this process, water is decomposed into its elements by passing an electric current through it. The electric current is provided by a battery or another source of electricity and the water needs to have an **electrolyte**, such as salt or acid, added to it to make it conductive¹. Two electrodes, usually made of metal or other conductive material, are inserted into the water and connected to the battery. The electrode connected to the positive terminal of the battery is called the **anode** and the one connected to the negative terminal is called the **cathode**¹. When the electric current flows through the water, hydrogen gas bubbles form at the cathode and oxygen gas bubbles form at the anode¹. The overall chemical reaction for electrolysis of water is:
2 H2O → 2 H2 + O2
Another way to break down H2 is by using **heat** to cause a reaction between hydrogen and oxygen that produces water and releases a large amount of energy. This reaction is called **combustion** or **oxidation** and can be ignited by a spark or a flame³. The reaction is very fast and explosive and can be dangerous if not controlled. The overall chemical reaction for combustion of hydrogen is:
2 H2 + O2 → 2 H2O
I hope this helps you understand how H2 can be broken down and what methods are used to do so.
Which of the atoms listed below has the largest radius?
A) AI
B) P
C) Si
D) Na
E) Mg
Answers
According to the given statement Na of the atoms listed below has the largest radius.
What is an atom?An atom is made up of a core nucleus and one or even more electrons with negative charges that orbit it. The positively charged, comparatively hefty protons and neutrons that make it up the nucleus may be present. The fundamental building components of matter are atoms.
How are atoms made?Atoms are made up of a nucleus in the center that is surrounded by protons, neutrons, and electron. Uranium is divided into smaller atoms during the fission process, creating new atoms. The creation or atoms in enormous numbers can be seen in the Big Bang and Supernova phenomena.
To know more about Atoms visit:
https://brainly.com/question/1566330
#SPJ13
QUESTION 5
How can you increase the kinetic energy of the particles in a substance?
Answers
Answer: A rise in temperature increases the kinetic energy and speed of particles; it does not weaken the forces between them. The particles in solids vibrate about fixed positions; even at very low temperatures. Individual particles in liquids and gases have no fixed positions and move chaotically.
Answer:
energy kinetic particles in substance
How to find ppt and name and find formula. Please and thank you

Answers
The precipitates are
Ppt A Sr(OH)2 Strontium hydroxide
Ppt B BaCO3 Barium carbonate
Ppt C SrCO3 Strontium carbonate
Ppt D BaSO4 Barium sulphate
Ppt E Sr(OH)2 Strontium hydroxide
Ppt F SrSO4 Strontium sulphate
What is a precipitate ?
Precipitation is the process of changing a dissolved substance from a super-saturated solution to an insoluble solid in an aqueous solution. Precipitate refers to the produced solid. The chemical agent that initiates the precipitation in an inorganic chemical process is referred to as the precipitant.
The term "supernate" or "supernatant" also refers to the clear liquid that remains on top of the precipitated or centrifuged solid phase.
The precipitates are
Ppt A Sr(OH)2 Strontium hydroxide
Ppt B BaCO3 Barium carbonate
Ppt C SrCO3 Strontium carbonate
Ppt D BaSO4 Barium sulphate
Ppt E Sr(OH)2 Strontium hydroxide
Ppt F SrSO4 Strontium sulphate
To know more about Precipitate from the given link
https://brainly.com/question/866725
#SPJ13
As the temperature of a gas increases, the volume of the gas will______ if the pressure remains the same
Answers
Answer:
increasesExplanation:
The volume of the gas increases as the temperature increases. As temperature increases, the molecules of the gas have more kinetic energyAnswer:
Explanation: stay the same
Which one of these statements about strong acids is true?
A) All strong acids have H toms bonded to electronegative oxygen atoms. Strong acids are 100% ionized in water.
C) The conjugate base of a strong acid itself a strong base
D) Strong acids are very concentrated acids.
E) Strong acids produce solutions with a higher pH than weak acids.
Answers
Answer:
Strong acids are 100% ionized in water.
Explanation:
A strong acid is any acid that is completely ionized in water (achieves 100% ionization). Weak acids do not ionize completely in water. Many weak acids ionize to a very small extent in pure water.
Examples of strong acids are HCl, H2SO4, HNO3, etc.
NEED HELP FIGURING HOW MANY MOL!! PLEASE QUICK!!THANK YOU SO MUCH

Answers
The number of moles of the gas by the ideal gas law is 0.18 moles.
What is the ideal gas law?The behavior of an ideal gas, a hypothetical gas made up of randomly moving particles with little volume and no intermolecular interactions, is described by the ideal gas law.
Although intermolecular interactions and non-zero particle volume prevent gases from always behaving in an ideal manner, the ideal gas law is nevertheless a good approximation for many gases under some circumstances.
We know that;
PV = nRT
We have ;
P = 1.2 atm
V = 3.4 L
T = 10 + 273 = 283 K
n = ?
n = PV/RT
n = 1.2 * 3.4/0.082 * 283
n =4.08 /23.2
n = 0.18 moles
Learn more about the ideal gas law:https://brainly.com/question/30458409
#SPJ1
am i gregnant? (im a guy)
how do i use a doorknob
Answers
uhm no and u turn it
Answer:
what does that mean
Explanation:
you just grab an axe and start hitting
Correct formula for barium hydroxide
Answers
Ba(OH)2
HOPE IT HELPS...!!!
1. Your teacher will show a video that demonstrates how aluminum metal reacts
with copper (II) chloride (CuCl₂). Answer the following questions about the
reaction you observed between aluminum and copper(II) chloride.
(a) Based on the observations you made during the video, write the balanced
molecular equation for the reaction between solid aluminum and a solution
of copper(II) chloride.
Answers
The balanced molecular equation for the reaction between solid aluminum and a solution of copper(II) chloride is: 2Al(s) + 3CuCl2(aq) → 3Cu(s) + 2AlCl3(aq).
What takes place when aluminium and copper II chloride interact?Aluminium and copper(II) chloride react very vigorously, causing the reaction mixture to become extremely hot as heat is produced, the aluminium foil to dissolve, a reddish brown solid to appear, and gas bubbles to be released.
What does place when aluminium foil is dipped in a copper II nitrate solution?The aluminium foil disintegrates, heat is released, the copper(II) ions' blue colour disappears, and a new, reddish-brown solid develops in the reaction mixture.
To know more about solution visit:-
https://brainly.com/question/30665317
#SPJ1
Whoever wrote that basic answer to my chemistry question that is NOT the complex equation. One of the reactants is [Cu(OH2)6]^2+
so what's the rest of the equation?
Answers
Here is the balanced chemical equation for the reaction involving \([Cu(OH_2)_6]^{2+}:\)
\([Cu(OH_2)_6]^{2+} + 4Cl^- = [CuCl_4]^{2-} + 6H_2O\)
In this reaction, \([Cu(OH_2)_6]^{2+}\) is a complex ion of copper(II) that reacts with chloride ions to form the complex ion \([CuCl_4]^{2-}\) and water molecules.
The balanced equation indicates that for every one mole of \([Cu(OH_2)_6]^{2+\) that reacts, four moles of chloride ions are required and two moles of \([CuCl_4]^{2-\) and six moles of water are produced.
A balanced chemical equation is a written representation of a chemical reaction that shows the same number of atoms of each element on both the reactant and product sides of the equation.
In other words, the law of conservation of mass is obeyed in a balanced chemical equation, which states that the total mass of the reactants must equal the total mass of the products.
For more question on balanced chemical equation click on
https://brainly.com/question/11904811
#SPJ11
Please help me 17 points!

Answers
Answer:
A dam: 3
solar cells: 2
food: 1
a rocket: 1
Answer:
1: yes
Explanation: