Answers
Based on the proportionality established by the given data, we predict that the force of gravity on an object of mass 1000 kg would be 50 Newtons.
To predict the force of gravity on an object of mass 1000 kg, we can use the concept of proportionality. Based on the given information, we have two data points: the mass of an object of 500 kg and the corresponding gravitational force of 25 Newtons.
Let's denote the force of gravity on the object of mass 1000 kg as F₁ and solve for it using a proportion:
mass₁ / force₁ = mass₂ / force₂
Plugging in the values we have:
1000 kg / F₁ = 500 kg / 25 N
Cross-multiplying and solving for F₁:
(1000 kg) * (25 N) = (500 kg) * F₁
25,000 kg*N = 500 kg * F₁
Dividing both sides by 500 kg:
F₁ = (25,000 kg*N) / 500 kg
F₁ = 50 N
For such more questions on gravity
https://brainly.com/question/18258780
#SPJ8
Related Questions
Infer: The metric system uses prefixes to tell you how much to multiply the base unit by. For example, in the metric system, the base unit for length is the meter. Examples of prefixes include kilo-, centi-, and milli-.
Knowing that 1,000 meters are in 1 kilometer, what do you think the prefix kilo- means?
Answers
Answer:
kilo means one thousand
Explanation:
Seeing as there are 1000 metres in one kilometre
Knowing that 1,000 meters are in 1 kilometer, The prefix kilo means 1000 or 1 kilogram (kg).
What is the metric system?The metric system is the system that uses gram, liter, and meter as the base unit for measuring the distance, volume, and length of any object or entity.
The metric system was started in France in 1970. There are common seven units in the metric system. The three main base units are gram, liter, and meter.
A liter is used to measure the Volume. Grams are used to measuring the quantity of a solid item. Meter is used to measure the distance or length of anything.
The kilogram is the base unit for measuring the quantity of any solid item, such as rice, and 1 kg of rice is the quantity of rice.
Thus, the prefix kilo means 1000 or 1 kilogram (kg).
To learn more about the metric system, refer to the link:
https://brainly.com/question/25966695
#SPJ2
What is the mass percentage of C in morphine, C₁7H19NOs? Provide an
answer to two decimal places.

Answers
The mass percentage of C in morphine would be 4.21%.
What is mass percentage?The mass percentage of a composition in a compound is the mass of the composition relative to the mass of the entire compound. This can be mathematically expressed as:
Mass percentage = mass of component/mass of substance x 100%
In this case, we are looking for the mass percentage of C in \(C_{17}H_{19}NO_3\).
Molar weight of C = 12 g/mol
Molar mass of \(C_{17}H_{19}NO_3\) = (12x17) + (1x19) + (14x1) + (16x3)
= 285 g/mol
Mass percentage of C = 12/285
= 4.21% to 2 decimal places.
In other words, the mass percentage of C in morphine is 4.21%.
More on mass percentage can be found here: https://brainly.com/question/16885872
#SPJ1
7. What is the volume of the
composite
solid?
4 in.
3 in.
3 in.

Answers
Answer:
The volume of Component 1 is 36 cubic inches.
Explanation:
To calculate the volume of a composite solid, we need to determine the individual volumes of the different components and then add them together.
In this case, the composite solid consists of multiple components with the following dimensions:
Component 1:
Length: 4 inches
Width: 3 inches
Height: 3 inches
To find the volume of Component 1, we multiply the length, width, and height together:
Volume of Component 1 = Length x Width x Height = 4 in x 3 in x 3 in = 36 cubic inches
Therefore, the volume of Component 1 is 36 cubic inches.
Please provide the dimensions of the remaining components of the composite solid, and I will calculate the total volume by summing up the individual volumes.
Express the answer to the following in scientific notation.
850,000 - 9.0x10^5
Answers
850,000 - 900,000 = -50,000
-50,000 = -5.0x10^4 = Answer
what element is this

Answers
Answer:
Nitrogen (N)
Explanation:
From the question given above, the following data were obtained:
Electronic configuration of the element => 1s² 2s²2p³
Next, we shall determine the number of electrons present in the element.
Thus, the number of electrons in the element can be obtained as follow:
Number of electron = 2 + 2 + 3
Number of electron = 7
Next, we shall determine the number of protons in the element.
Since the element is neutral, the number of protons is the same as the number of electrons. Mathematically,
Number of protons = number of electrons
Number of electron = 7
Therefore,
Number of protons = 7
Finally, we shall determine the atomic number of the element.
The atomic number of an element is the number of protons in the atom of the element. Mathematically,
Atomic number = number of protons
Number of protons = 7
Therefore,
Atomic number = 7
Comparing the atomic number of the element ( i.e 7) with those in the periodic table, the name of the element is Nitrogen (N) since no two elements have the same atomic number.
if 650J heat is absorbed by a system and 450J work is done on the system, then find the change in internal energy of the system
Answers
Answer: 380 J. Please mark
Explanation:
What is the percent composition of each element in CaCO 3 ? ( Mass of Element divided by mass of Compound) x 100
Answers
Answer:
molar mass of CaCO3=40+12+3(16)
=100 gmol-1
Ca percent composition=40/100*100%
=40%
C percent composition=12/100*100%
=12%
0 percent composition=48/100*100%
=48%
Answer:
Since we're sticking with theoretical values instead of experimental, Percent Composition would be (Molar Mass of the element) / (Molar mass of compound) x 100%
Molar mass of Calcium: 40g/mol
Molar mass of Carbon: 12g/mol
Molar mass of Oxygen: 16g/mol
Molar mass of CaCO3 (Calcium Carbonate) = (40) + (12) + (16)*3 = 100g/mol
Percentage composition of Calcium = \(\frac{40}{100} \ * \ 100 =\) 40% of Calcium
Percentage composition of Carbon = \(\frac{12}{100} \ * \ 100 =\) 12% of Carbon
(For the next one, there are 3 oxygen, so it'd be 16x3 = 48g/mol)
Percentage composition of Oxygen = \(\frac{48}{100} \ * \ 100 =\) 48% of Oxygen
Wireless Internet networks, including many used in homes, often make use of high-frequency radio waves. High-frequency waves are useful because they can carry a lot of information. However, high-frequency waves are less capable of passing through objects than are low-frequency waves. As a result, waves traveling from a person's wireless laptop computer, for example, could be interrupted by objects between the computer and the modem.
Due to this limitation of high frequency waves, which of the following statements best explains why digital waves are commonly used in high-frequency wireless networks instead of analog waves?
Answers
Digital waves are commonly used in high-frequency wireless networks instead of analog waves because digital signals are less susceptible to interference from obstacles and noise.
Analog signals vary continuously over time and can be affected by various forms of interference, such as distortion or attenuation, which can result in the loss or corruption of information.
In contrast, digital signals are represented as a series of discrete values or binary digits, which are more resilient to interference and can be easily reconstructed at the receiving end. Digital signals are also easier to compress, allowing for more efficient use of the available bandwidth and higher data transfer rates.
Therefore, digital signals are the preferred choice for high-frequency wireless networks as they provide reliable, high-speed data transmission while minimizing the impact of interference and signal loss caused by obstacles.
For more such questions on Digital waves
https://brainly.com/question/16428806
#SPJ11
Note : The search engine could not find the complete questions.
What noble gas does the element bismuth want to gain 3 electrons to become?
Answers
Answer:
Explanation:
Nitrogen and Phosphorus
Which of the following describes a PHYSICAL PROPERTY? *
3 points
A two liquids mix to produce a solid
B alcohol is flammable
C silver can be stretched into thin wires
D milk spoils
Answers
Answer:
silver can be stretched into thin wires.
4. Calculate the final volume of a gas if it's initial pressure is 118 kPa and original volume is 17 Liters, and the final pressure is recorded as 265 kPa.
Answers
Answer:
7.57 LExplanation:
The new volume can be found by using the formula
\(V_2 = \frac{P_1V_1}{P_2} \\\)
where
P1 is the initial pressure
P2 is the final pressure
V1 is the initial volume
V2 is the final volume
From the question we have
\(V_2 = \frac{118 \times 17}{265} \\ = 7.569811\)
We have the final answer as
7.57 LHope this helps you
Calculate the free energy change for the hydrolysis of ATP under these conditions.
The standard free energy change for the hydrolysis of ATP is -46.5 kJ.
ATP(aq)+H2O(l)→ADP(aq)+Pi(aq)
The concentrations of ATP, ADP, and Pi are 0.0089 M, 0.0019 M, and 0.0038 M. (Assume a temperature of 298 K.)
Answers
The free energy change for the hydrolysis of ATP by using the equation ATP(aq)+H2O(l)→ADP(aq)+Pi(aq) is -64362.77joules.
How do we calculate free energy change of reaction?We can calculate the free energy change of reaction by using the standard free energy by using the below link:
ΔG = ΔG⁰ + RTlnQ, where
R = ideal gas constant = 8.314 J/mol.K
ΔG⁰ = standard free energy = -46.5 kJ = -46500 J
T = temperature = 298 K
Q = ratio of the products to reactants for the given chemical reaction
Q = [0.0019][0.0038] / [0.0089] = 8.1×10⁻⁴
On putting values on the above equation, we get
ΔG = -46500 + (8.314)(298)ln(8.1×10⁻⁴)
ΔG = -46500 + (2477.5)(-7.21)
ΔG = -46500 - 17862.77
ΔG = -64362.77
Hence required value of free energy change is -64362.77 joules.
To know more about free energy change, visit the below link:
https://brainly.com/question/15876696
#SPJ1
Two parents who are both Gg (Bb) for a gene are crossed. The genetic makeup of the offspring is that some will be Gg and some gg.
Question 2 options:
True
False
Answers
Answer:
false
Explanation:
The genotype of 50% of offspring will be Gg, 25% will be gg, and 25% will be GG. Hence, it is true.
What is a genotype?An organism's genotype is made up of all of its genetic components. The alleles or variations that an individual carries in a specific gene or genetic region are also referred to as the genotype. In other words, it is the genetic makeup of an organism. Phenotypes are determined by genotypes.
There are three different genotype types: homozygous dominant (PP), homozygous recessive (PP), and heterozygous (Pp). Examples of genotypes are hair color, skin color, height, hair texture, and blood group.
When two parents who are both Gg (Bb) for a gene are crossed, then the genotype of their offspring will be GG, gg, Gg. Hence, it is true.
Learn more about genotype, here:
https://brainly.com/question/26124553
#SPJ6
The solubility of sugar in water__________with an increase in temperature
Answers
Answer:
increase
Explanation:
When temperature increases the kinetic energy of the particles in the sugar increases causing the to collide more frequently with the water particles thereby increasing the rate of solubility
For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M, and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant (Kc) for the given reaction is approximately 16.448. The value of Kc indicates the relative concentrations of reactants and products at equilibrium. In this case, a Kc greater than 1 suggests that the products (H2 and P4) are favored at equilibrium, indicating that the forward reaction is more favorable.
To determine the equilibrium constant (Kc) for the given reaction:
4PH3(g) ↔ 6H2(g) + P4(g)
We can write the equilibrium constant expression based on the stoichiometric coefficients:
Kc = ([H2]^6 * [P4]) / ([PH3]^4)
Substituting the given equilibrium concentrations:
[PH3] = 0.250 M
[H2] = 0.580 M
[P4] = 0.750 M
We can plug in these values into the equilibrium constant expression:
Kc = ([0.580]^6 * [0.750]) / ([0.250]^4)
Kc = (0.0860128 * 0.750) / (0.00390625)
Kc = 16.448
for more question on equilibrium
https://brainly.com/question/18849238
#SPJ8
True or False Polyunsaturated fatty acids are precursors of other molecules.
Answers
Dietary fats include polyunsaturated fat. Along with monounsaturated fat, it is one of the good fats. Foods like salmon, vegetable oils, and other plant- and animal-based foods include polyunsaturated fat. They act as precursors of other molecules. The given statement is true.
Precursors of eicosanoids with hormone-like properties including prostaglandins, leukotrienes, and thromboxanes are polyunsaturated fatty acids (PUFA).
Dihomo-linolenic acid (DGLA), arachidonic acid (AA), and eicosapentaenoic acid (EPA) are the three precursors that prostaglandins can be formed from. Depending on the precursor, series 1, 2, or 3 prostanoids is produced.
To know more about Polyunsaturated fatty acids, visit;
https://brainly.com/question/12409149
#SPJ1
When 0.717 g of sodium metal is added to an excess of hydrochloric acid, 7450 J of heat are produced. What is the enthalpy of the reaction as written?
2Na(s)+2HCl(aq)⟶2NaCl(aq)+H2(g)
Answers
Answer:
The enthalpy change of the reaction is 4.78 × 10⁴ J.
Explanation:
We are given that 0.717 g of sodium metal reacts with hydrochloric acid to produce 7450 J of heat.
Converting grams of sodium to moles:
\(\displaystyle 0.717 \text{ g Na} \cdot \frac{1 \text{ mol Na}}{22.99 \text{ g Na}} = 0.0312 \text{ mol Na}\)
And dividing the amount of heat produced by the moles of sodium reacted yields:
\(\displaystyle \Delta H = \frac{7450 \text{ J}}{0.312 \text{ mol Na}} = 2.39 \times 10^4 \text{ J/mol Na}\)
Because the given reaction has two moles of sodium metal, we can multiply the above value by two to acquire the enthalpy change of the given reaction:
\(\displaystyle \Delta H = 2\text{ mol Na}\left(2.39 \times 10^4 \text{ J/mol Na}}\right) = 4.78 \times 10^4 \text{ J}\)
In conclusion, the enthalpy change of the reaction is 4.78 × 10⁴ J.
The empirical formula of p-dichlorobenzene is C3H2Cl. What is the molecular formula if its molar mass is 147 g/mol? Pls help I will give brainliest.
Answers
The molecular formula of p-dichlorobenzene is C6H4Cl2.
What is an empirical formula?The simplest whole-number ratio of the atoms in a compound is an empirical formula. In other words, it shows the relative number of atoms of each element in a compound, but not the actual number of atoms or the molecular structure. Empirical formulas are often determined by experimental data, such as the mass percentages of each element in the compound, and they are useful for comparing the compositions of different compounds.
The molar mass of the empirical formula can be calculated as follows:
C3H2Cl:
3C = 3 x 12.01 g/mol = 36.03 g/mol
2H = 2 x 1.01 g/mol = 2.02 g/mol
1Cl = 1 x 35.45 g/mol = 35.45 g/mol
Total molar mass = 73.50 g/mol
Now we can calculate the molecular formula by dividing the molar mass of the compound by the molar mass of the empirical formula, and then multiplying each subscript in the empirical formula by this number:
Molecular mass / empirical mass = 147 / 73.50 = 2
C3H2Cl x 2 = C6H4Cl2
To know more about experimental data, visit:
https://brainly.com/question/8828700
#SPJ1
If 2.12 Moles of bromine react with excess phosphorus; How many moles of phosphorus tribromide can be produced?
Round all answers to the hundredths place.
Answers
6 molls
As a result, if two moles of dinitrogen gas are specified, six moles of dihydrogen gas are needed.
Which molecule is butane?
H H H H
A. H-C-C-C-C-H
||||
H H H H
B.
C.
H3C
C=C
H
CH3
H
H
|
D. H-C=C-C-C-H
H H
H
|
Answers
Answer: A
Explanation:
The -ane suffix implies that the compound has only single bonds for carbon-carbon bonds. The but- prefix implies that the compound consists of four carbons. Since 4 bonds are required for each carbon, there will be a total of 10 hydrogen atoms: 3 on each carbon at the end of the chain and 2 for each carbon in the middle of the chain. Thus, butane is A.
A 2.50M solution of NaOH is diluted from a volume of 0.890L to a volume of 2.00L, What is the new molarity?
O 2.78011
O 1.11M
O 5.62M
O 4.5M
Answers
Answer:
1.11 M
Explanation:
M₁V₁ = M₂V₂
Step 1: Define
Molarity₁ = 2.50 M
Volume₁ = 0.890 L
Molarity₂ = unknown
Volume₂ = 2.00 L
Step 2: Substitute and Evaluate for M₂
(2.50 M)(0.890 L) = (M₂)(2.00 L)
2.225 = M₂(2.00 L)
M₂ = 1.1125 M
Step 3: Simplify
We are given 3 sig figs.
1.1125 M ≈ 1.11 M
If a certain gas occupies a volume of 12 LL when the applied pressure is 6.0 atmatm , find the pressure when the gas occupies a volume of 3.0 LL . Express your answer to two significant figures, and include the appropriate units. View Available Hint(s)
Answers
Given :
If a certain gas occupies a volume of 12 LL when the applied pressure is 6.0 atm.
To Find :
The pressure when the gas occupies a volume of 3.0 LL .
Solution :
Since, their is no information of temperature, let us assume it is constant throughout the process.
We know, by ideal gas equation at constant temperature :
\(\alpha P_1V_1 = P_2V_2\\\\6 \times 12 = P_2 \times 3\\\\P_2 = 24\ atm\)
Therefore, the pressure when the gas occupies a volume of 3.0 LL is 24 atm.
The chemical equation below shows the reaction between carbon dioxide (CO2) and lithium hydroxide (LiOH). CO2 + 2LiOH Right arrow. Li2CO3 + H2O The molar mass CO2 is 44.01 g/mol. How many moles of LiOH are needed to react completely with 25.5 g of CO2? 0.290 moles 0.579 moles 1.16 moles 1.73 moles
Answers
As per the given reaction, 2 moles or 46 g of LiOH is needed to completely react with CO₂. Then, 25.5 g of CO₂ requires 1.16 moles of LiOH.
What is lithium hydroxide ?Lithium hydroxide is an ionic compound formed by the combination of metallic lithium and water moiety. LiOH easily reacts with carbon dioxide forms an insoluble compound lithium carbonate and water.
As per the given balanced equation of the reaction between LiOH and carbon dioxide, one mole of CO₂ reacts with 2 moles of LiOH.
molar mass of CO₂ = 44 g/mol
molar mass of LiOH = 23 g/mol
mass of 2 LiOH = 46 g.
Then, mass of LiOH needed to react with 25 .5 g of CO₂ = (25.5 × 46) /44 = 26.13 g
Number of moles in 26.13 g of LiOH = 26.13/23 = 1.16 moles.
Therefore, the number of moles of LiOH are needed to react completely with 25.5 g of CO2 is 1.16 moles.
Find more on LiOH:
https://brainly.com/question/16251002
#SPJ1
11. What is deceleration also called?
negative velocity
negative acceleration
negative speed
positive stopping
Answers
Explanation:
it is negative acceleration
Answer:
negative acceleration
Explanation:
Which if the following matters occupies more space, assuming similar number of molecules?
Answers
Assuming a similar number of molecules, the matter that occupies the most space is gas. Option C is correct.
This is because gases have no definite shape or volume, and their molecules are spread out, moving freely in all directions. As a result, gases tend to occupy the entire volume of their container and expand to fill the available space. This is known as the "kinetic molecular theory" of gases.
In contrast, solids and liquids have a definite volume and shape. Solids have a fixed shape and their molecules are packed closely together, while liquids have a variable shape and their molecules are less closely packed. As a result, both solids and liquids occupy less space than gases.
It is worth noting that the volume of a solid or liquid can change under certain conditions, such as changes in temperature or pressure. However, even under these conditions, the space occupied by a solid or liquid is still less than that occupied by a gas. Option C is correct.
The complete question is
Which of the following matters occupies more space, assuming similar number of molecules?
A. Solid
B. Liquid
C. Gas
D. Solid and gas
To know more about the Matter, here
https://brainly.com/question/30028447
#SPJ1
can someone help me pls

Answers
Answer:
I dont understand what the question means
Explanation:
The electronegativity is 2.1 for H and 3.0 for Cl. Based on these electronegativities, HCI would be expected to A. have polar covalent bonds with a partial positive charges on the H atoms. B. have ionic bonds and contain H+ ions. C. have nonpolar covalent bonds and contain Hions. D. have nonpolar covalent bonds with a partial negative charges on the H atoms.
Answers
Based on these electronegativities, HCI would be expected to have polar covalent bonds and contain H+ ions.
Hydrochloric acid, also referred to as muriatic acid, is HCl. It is a component of the hydrogen halides (HX) family. Due to their propensity to lose a proton in a solution.Hydrogen halides are diatomic inorganic molecules that function as Arrhenius acids. Since the X belongs to the halogen family (group 17), it can be fluorine, chlorine, bromine, or iodine. In hydrogen chloride, only 17 percent of the hydrogen atom's electron density has been transferred to the chlorine atom. It confirms that the H-Cl bond in the hydrogen chloride is a polar covalent bond, not an ionic bond.In hydrogen chloride, the chlorine atom is 127.4 x 10⁻¹² m away from the hydrogen atom, giving the compound a 1.05 D dipole moment.1 D equals 3.33 x 10⁻³⁰ C. m.The chlorine atom i will have an equal but opposite charge. e. Therefore, the value of the partial charge in the hydrogen chloride compound is 0.027 x 10⁻¹⁸ C.Mathematically,
% of ionic character = 16(Xa - XB ) + 3.5(XA - Xb )
=16(3-2.1)+3.5(3-2.1)²
= 14.4+2.835
= 17.235%
:. The nature of HCI is 17.2% ionic
To learn more about polar covalent bonds -
https://brainly.com/question/28295504
#SPJ4
Dinitrogen tetroxide in its liquid state was used as one of the fuels on the lunar lander for theNASA Apollo missions. In the gas phase it decomposes to gaseous nitrogen dioxide:N2O4(g) ↔ 2NO2(g)Consider an experiment in which gaseous N2O4 was placed in a flask and allowed to reachequilibrium at a temperature where Kp = 0.133. At equilibrium, the pressure of N2O4 wasfound to be 2.71 atm. Calculate the equilibrium pressure of NO2(g).
Answers
Equilibrium pressure of NO2 (g) is 0.3604. This is calculated from the expression of equilibrium constant.
Dinitrogen tetroxide in its liquid state was used as one of the fuels on the lunar lander. Decomposition of gaseous nitrogen dioxide ,
N2O+ (g) ---> 2NO2 (g)
chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time. For this equilibrium reaction, equilibrium constant can be represented as
K(eq.) = concentration of product / concentration of reactant
In gaseous phase we consider the presence of the molecule ,
Kp = [NO2] / [N2O4]
where, Kp = equilibrium constant that equals to 0.133
[N2O4] = Presence of N2O4 at equilibrium that equals to 2.71.
Putting the values in the expression of equilibrium constant,
0.133 = [NO2]2 / 2.71
[NO2]2 = 0.133 * 2.71
= 0.3604
To learn more about Equilibrium constant please visit:
https://brainly.com/question/3159758
#SPJ4
The above graph shows the journey of squirrel for a certain interval. Which of the following describes the motion of the squirrel between 5 s and 8 s?
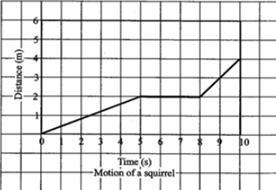
Answers
The statement that describes the motion of the squirrel between 5s and 8s is as follows: The squirrel's speed did not change (option C).
What is a graph?A graph in mathematics and statistics is a data chart (graphical representation of data) intended to illustrate the relationship between a set (or sets) of numbers (quantities, measurements or indicative numbers) and a reference set, whose elements are indexed to those of the former set(s) and may or may not be numbers.
According to this question, a graph is used to illustrate the relationship between the distance moved by a squirrel and the time it took it to move i.e. the journey of squirrel for a certain interval or period of time.
Based on the graph above, the speed increased with time up until 5 seconds, however, from 5seconds till 8 seconds, the speed of the squirrel remained constant i.e. did not change until it picked up again.
Therefore, option C is the correct description of the situation of the squirrel between 5s to 8s.
The options to the incomplete question are as follows:
A. The squirrel's speed increased
B. The squirrel's speed decreased
C. The squirrel's speed did not change
D. The squirrel moved backward
Learn more about graph at: https://brainly.com/question/17267403
#SPJ1
If [H3O^ + ]=1.7*10^ -8 M what is the pOH of the solution?
Answers
Answer: 6.23
Explanation:
1) solve for pH
pH=-log (H3O+) = - log 1.7 X 10^-8 =7.77
2) now do 14-pH = 14 -7.77=6.23