Add a spoonful of sugar to one
cup of water. Add a spoonful of
oil to a second cup of water. Stir
each cup. Record your
observations below.
Answers
Answer:
The sugar will dissolve in water.
The oil will float in water.
Explanation:
The sugar molecules will fill up the remaining space between the molecules of water.
The oil being lighter and less denser than water floats in the water.
Please mark as brainliest
Related Questions
I would like to know if question number 3 is rite

Answers
The balanced reaction of this chemical reaction is as follows :
\(2HClO_4(aq)\text{ + Ba}(OH)_2(aq)\text{ }\Rightarrow Ba(ClO_4)_2(aq)+2H_2O(l)\text{ }\)• As we can see, this is a double-displament reaction
The equation for the burning of hydrogen is:
2H2(g) + O2(g) → 2H2O(g)
One mole of hydrogen gas is mixed with one mole of oxygen gas and burnt. What will
be present after the reaction?
Answers
The amino acid glycine can be condensed to form a polymer called polyglycine. Draw the repeating monomer unit.
Answers
The repeating monomer unit of polyglycine is shown below:
\(H_{2}N-CH_{2}-CO-\\\\\)
The repeating monomer unit of polyglycine is simply the amino acid glycine itself. This represents one glycine molecule, with an amine group at one end and a carboxylic acid group at the other end. When glycine molecules are linked together through peptide bonds, the amine group of one molecule reacts with the carboxylic acid group of another, releasing a molecule of water and forming a peptide bond (-CO-NH-). This process is repeated to form the polymer polyglycine.
When glycine monomers undergo condensation polymerization, the carboxyl group of one glycine molecule reacts with the amino group of another glycine molecule, forming a peptide bond and releasing a water molecule. This process is repeated for each additional glycine monomer that is added to the growing polymer chain.
Learn more about carboxylic acid here:
https://brainly.com/question/31377595
#SPJ11
if 2.5 moles of zinc react with 6.0 moles of hydrochloric acid Zn + 2HCl → ZnCl2 + Hz, find mass of zinc chloride . what is the limiting reactant Zn (A=65.3) Cl (A=35.5) ? b) 136g c) 160g d) 165 g a) 140 g
Answers
Answer:
The mass of zinc chloride produced when 2.5 moles of zinc react with 6.0 moles of hydrochloric acid is 165 g. The limiting reactant is zinc, since it is the reactant with the smallest amount.
Explanation:
As a consequence, 2.5 moles of ZnCl2 weigh 340g. Because we have 2.5 moles of Zn, it is the limiting reactant. 140g is the right answer.
What is limiting reactant?In a chemical process, the limiting reagent is a reactant that is completely consumed when the reaction is complete. This reagent limits the amount of product generated since the reaction cannot continue without it.
Here,
a) Calculate the mass of zinc chloride produced as follows:
1 mole of ZnCl2 weighs 65.3 + 2 * 35.5 = 136g.
As a result, 2.5 moles of ZnCl2 have a mass of 2.5 * 136 = 340g.
b) To determine the limiting reactant, we compare the amount of each reactant to the amount required to react with all other reactants.
6 moles * 2 moles Zn/2 moles HCl = 6 moles Zn are required to react with all of the hydrochloric acid.
Zn is the limiting reactant since we have 2.5 moles of it.
d) The correct answer is 140g.
As a result, 2.5 moles of ZnCl2 have a mass of 340g. Zn is the limiting reactant since we have 2.5 moles of it. The correct answer is 140g.
To know more about limiting reactant,
https://brainly.com/question/28945068
#SPJ4
when a nucleus emits an alpha particle, the mass number of the nucleus?
a. inrease and its at. number decreases
b. decreases and its at. number decreases
c. decreases and its at. number increases
d. remainds smae and its at. number decreases
Answers
When a nucleus emits an alpha particle, the mass number of the nucleus decreases, and its atomic number also decreases.
Therefore, the correct answer is: (c). The mass number decreases and its atomic number increases.
The nuclei are incredibly tiny and dense. They are 10 thousand times smaller than an atom and have more than 99.9% of its mass! Protons, which have a positive charge, and neutrons, which have no electrical charge, make up the nucleus.
When a nucleus emits an alpha particle, the mass number of the nucleus decreases, and its atomic number also decreases.
An alpha particle is composed of two protons and two neutrons, which is equivalent to a helium nucleus (He²⁺). When an alpha particle is emitted from a nucleus, the mass number decreases by 4 (as the alpha particle has a mass of 4 atomic mass units) and the atomic number decreases by 2 (since the nucleus loses two protons).
Therefore, the correct answer is:
c. The mass number decreases and its atomic number increases.
To know more about alpha particle:
https://brainly.com/question/24276675
#SPJ4
Nuclear fission occurs when _______________ a. TNT and plutonium are combined, causing the molecules to separate. b. a nucleus breaks up into two equal fragments that release and separate more atoms. c. like atoms collide to create double nuclei. d. trinitite is created by multiple molecules that form a single atom.
Answers
Nuclear fission occurs when a nucleus breaks up into two equal fragments that release and separate more atoms. So, the correct option is B.
Nuclear fission is a process in which the nucleus of an atom breaks apart into two or more smaller nuclei. This process releases a significant amount of energy.
Option B accurately describes the process of nuclear fission. When a heavy nucleus, such as uranium-235 or plutonium-239, absorbs a neutron, it becomes unstable and splits into two smaller nuclei.These smaller nuclei, along with additional neutrons, are released in the process. The release of neutrons can trigger a chain reaction, where each neutron released can potentially collide with other nuclei, causing them to undergo fission as well.The energy released during nuclear fission is due to the conversion of a small amount of mass into a large amount of energy, as described by Einstein's famous equation, E=mc².This energy is utilized in various applications, including nuclear power generation and nuclear weapons. Nuclear fission reactions are carefully controlled in nuclear power plants to ensure the sustained release of energy without leading to uncontrolled chain reactions. Hence the correct option is B.
For more questions on nucleus
https://brainly.com/question/29855834
#SPJ8
Answer the questions about the characteristics of the elements in group 1 (the alkali metals). What happens when the elements in group 1 react with bromine? No reaction a salt is formed with the general formula MBr2 a salt is formed with the general formula MBr What happens when the elements in group 1 react with water? Hydrogen gas is released no reaction What happens when the elements in group 1 react with oxygen? No reaction an oxide is formed with the general formula MO an oxide is formed with the general formula M2O Which group 1 element reacts the most vigorously? Na Rb Li K Cs Which group 1 element exhibits slightly different chemistry from the others? Li Na Cs K Rb
Answers
Answer:
See explanation
Explanation:
The elements in group form univalent positive ions and element in group 17 form univalent negative ions. Hence, when a group 1 element reacts with a group 17 element, a compound of the sort MX is formed. Hence, when a group 1 element reacts with bromine, a salt is formed with the general formula MBr.
Elements of group 1 are highly electro positive metals. They react with water to form the metal hydroxide and release hydrogen gas. Hence, when group 1 elements react with water, hydrogen gas is released.
A group 1 element forms a univalent positive ion while a group 16 element forms a divalent negative ion. Hence, when a groups 1 element reacts with oxygen, the compound formed must have the general formula M2O.
The reactivity of group 1 metal increases down the group hence Cs is the most reactive group 1 element.
Lithium displays a slightly different chemistry from other group 1 elements because of its small size.
Explain how the tasks an organism completes will influence their cellular respiration levels.
Answers
Answer:
All living things use cellular respiration to turn organic molecules into energy. ... This process makes energy from food molecules available for the organism to carry out life processes. Cellular respiration usually occurs in the presence of oxygen. This is called aerobic respiration.
why does the hydrogen gas need to flow continuously for a while before starting the heating process?
Answers
In the laboratory, hydrogen gas is used as fuel for various purposes, including heating. In order to start the heating process, it is necessary to allow the hydrogen gas to flow continuously for a while. This is because there may be air or other gases present in the hydrogen gas pipeline that can affect the heating process.
When the hydrogen gas is allowed to flow continuously for a while, the air or other gases are purged from the pipeline, which improves the quality of the hydrogen gas. This ensures that there is no interference with the heating process, which could otherwise lead to inaccurate results.The continuous flow of hydrogen gas is essential because if it is not allowed to flow for a while, air or other gases can cause damage to the burner or other equipment used for heating. The air or other gases can cause an explosion, which can result in severe injury or death.In conclusion, the hydrogen gas needs to flow continuously for a while before starting the heating process to remove any air or other gases from the pipeline. This improves the quality of the hydrogen gas, ensures accurate results, and prevents damage to the equipment. It is important to follow safety protocols when using hydrogen gas to prevent any accidents.For such more question on heating process
https://brainly.com/question/29317333
#SPJ8
What is the pH of a solution with a [OH-] of 7.1 x 10-9?
W
Answers
Answer:
pH = 5.9
Explanation:
The equation to find pH is as follows:
pH = -log[H+]
We are not given [H+] so we have to find it given the value of [OH-].
To find [H+] from [OH-], use this equation:
\([H^{+} ]=\frac{1.0*10^{-14} }{[OH^{-}] }\)
Plug in [OH-] and solve for [H+]:
\([H^{+} ]=\frac{1.0*10^{-14} }{7.1*10^{-9} }\)
[H+] = \(1.41 * 10^{-6}\)
Now that we have found [H+] plug it into the pH equation and solve:
\(pH =-log(1.41*10^{-6} )\)
pH = 5.9
So, the pH of the solution would be 5.9
Hope this helps!! Ask questions if you need!
How do particles combine to form the variety of matter one observes?
Answers
Matter are anything that is made up of atoms. The quantity of matter can be observed only on the basis of mass and volume calculation. Therefore, by transferring electron or sharing electrons, particles combine to form the variety of matter.
What is matter?Matter is a substance that has some mass and can occupy some volume. The matter is mainly used in science. Matter can be solid, liquid or gas.
So as we saw that matter has some mass so mass can be measured in gram only. Mass can also be represented as number of molecules. We also saw that matter occupy some volume and that volume is measured only in liter. By transferring electron or sharing electrons, particles combine to form the variety of matter.
Therefore, by transferring electron or sharing electrons, particles combine to form the variety of matter.
To learn more about matter, here:
https://brainly.com/question/4562319
#SPJ1
Water is dripping into a bucket at a rate of 51 drops per minute. if each drop has a volume of 0.050 milliliters, what volume of water will drip into the bucket in 44 minutes?
Answers
112.2 milliliters volume of water will drip into the bucket in 44 minutes.
What is volume?How much space an object or substance takes up. • Measured in cubic meters (m3), liters (L) & milliliters (mL).
Total drop = Drops per minute X time
= 51 drops per minute X 44 minutes
=2244 drop
Volume of water will drip into the bucket in 44 minutes
=Total drop X Volume of each drop
=0.050 milliliters X 2244 drop
=112.2 milliliters
Hence, 112.2 milliliters volume of water will drip into the bucket in 44 minutes.
Learn more about volume here:
https://brainly.com/question/10904074
#SPJ1
Which career qualification is unique to the Energy Transmission career pathway and not to the Energy Distribution pathway
Answers
The career qualification that is unique to the Energy Transmission pathway and not to the Energy Distribution pathway is the knowledge and expertise in high-voltage power transmission.
Energy Transmission involves the bulk transportation of electricity at high voltages, over long distances, while Energy Distribution involves the delivery of electricity to end-users at lower voltages through local networks. Professionals in the Energy Transmission pathway need to have a deep understanding of high-voltage equipment and systems, including transformers, transmission lines, and substation infrastructure.
This expertise is critical in ensuring the safe and reliable transmission of large amounts of electricity across the grid. Therefore, a career in Energy Transmission requires specialized training and certification in high-voltage power transmission, which is not required in the Energy Distribution pathway.
More on energy: https://brainly.com/question/716903
#SPJ11
Oxidation often removes ...?
Answers
Answer:
Electrons.
Explanation:
Answer:
oh yes chemical oxidation removes
Explanation:
but i m not sure hope it is right i haven't looked book this time
A tank held neon gas at a pressure of 450 KPA, helium at a pressure of 175KPA and argon at a pressure of 210 kpa. what was the total pressure in the tank
Answers
To find the total pressure in the tank, we need to add up the pressures of the individual gases.
Total pressure = pressure of neon + pressure of helium + pressure of argon
Total pressure = 450 kPa + 175 kPa + 210 kPa
Total pressure = 835 kPa
Therefore, the total pressure in the tank was 835 kPa.
2NaCl is a....
(Mulititple choice)

Answers
Answer:
Coffeicent
Explanation:
I think this would help you
which of the following aqueous solutions has the lowest freezing point and the highest boiling point? which of the following aqueous solutions has the lowest freezing point and the highest boiling point? 0.050 m nacl 0.100 m sucrose 0.050 m cacl2 0.05 m na3po4
Answers
Of the four aqueous solutions listed, the one with the lowest freezing point and the highest solution boiling point is 0.050 M NaCl. This is because NaCl is a strong electrolyte, meaning it dissociates completely into ions in solution, and thus has the greatest effect on lowering the freezing point and raising the boiling point compared to the other solutions, which are weaker electrolytes or nonelectrolytes.
About SolutionsSolution is a homogeneous mixture consisting of two or more substances. The substance which is present in a smaller amount in a solution is called the solute or solute, while the substance which is present in a greater amount than the other substances in the solution is called the solvent or solvent.
Learn More About Solutions at https://brainly.com/question/3184550
#SPJ4
8. Fill in the blanks underneath the wave spectrum to indicate the relative positions of each type of
electromagnetic radiation from the given word bank.
gamma
ultraviolet
visible
X-rays
microwaves
infrared
radio/tv
wwwwww
annu
WNNNNN

Answers
The order of the radiations from longer wavelength to shorter wavelength is as follows: Radio waves , microwave, infrared, visible light, ultraviolet, X-rays and gamma rays. In these order the blanks have to be filled.
What is electromagnetic spectrum?Electromagnetic spectrum is the arrangement of radiations in the order of decreasing wavelength or increasing frequency. Frequency is the number of wave cycles per unit time.
All the electromagnetic radiations are transverse waves. Wavelength of an electromagnetic radiation is the distance between two consecutive crests or troughs or the waves.
As the wavelength increases frequency and energy of electromagnetic radiations decreases. The longest wave is radio waves.Shortest wave is gamma rays.
The given image of wave is from longer wavelength to shorter wavelength hence the radiations can be arranged as : Radio waves , microwave, infrared, visible light, ultraviolet, X-rays and gamma rays.
To find more on electromagnetic radiation, refer here:
https://brainly.com/question/10759891
#SPJ2
multiple choice question a solution contains 25.0 g ethanol (c2h5oh; molar mass 46.07 g/mol) in 500. g h2o (molar mass 18.02 g/mol) at 23oc. if the vapor pressure of pure h2o at this temperature is 20.57 torr, what is the vapor pressure of the solution? multiple choice question. 20.1 torr 0.390 torr 21.0 torr 0.979 torr
Answers
To calculate the vapor pressure of the solution, we need to use Raoult's law,
which states that the vapor pressure of a solution is equal to the mole fraction of the solvent times its vapor pressure in the pure state.
The mole fraction of water in the solution can be calculated as follows:
moles of water = mass of water / molar mass of water
moles of water = 500 g / 18.02 g/mol = 27.7 mol
moles of ethanol = mass of ethanol / molar mass of ethanol
moles of ethanol = 25.0 g / 46.07 g/mol = 0.543 mol
total moles of solution = moles of water + moles of ethanol
total moles of solution = 27.7 mol + 0.543 mol = 28.2 mol
mole fraction of water = moles of water / total moles of solution
mole fraction of water = 27.7 mol / 28.2 mol = 0.982
The vapor pressure of pure water at 23°C is given as 20.57 torr. Therefore, the vapor pressure of the solution can be calculated as:
vapor pressure of solution = mole fraction of water x vapor pressure of pure water
vapor pressure of solution = 0.982 x 20.57 torr
vapor pressure of solution = 20.18 torr
Therefore, the vapor pressure of the solution is 20.18 torr. The correct answer is 20.1 torr, which is the closest option given in the multiple-choice question.
learn more about vapor here: brainly.com/question/30820393
#SPJ11
Please dont answer if you dont know.
It's the parts of a wave
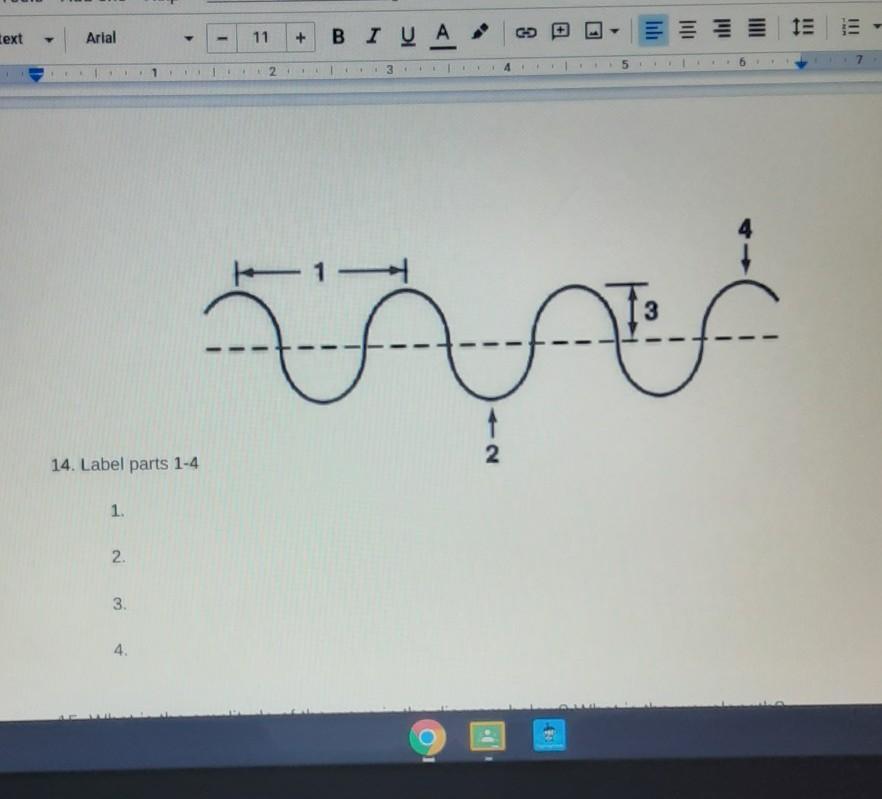
Answers
1) wavelength
2) trough
3) amplitude
4) crest
Explanation:
Hope this helps!
Calculate the mass of diphosphorus pentoxide (P2O5) that contains a million (1.00*10^6) phosphorus atoms. Be sure your answer has a unit symbol if necessary, and round it to 3 significant digits.
Answers
The mass of diphosphorus pentoxide (P2O5) that contains a million (1.00*10^6) phosphorus atoms is 0.118 g.
To determine the mass of diphosphorus pentoxide (P2O5) that contains a million (1.00*10^6) phosphorus atoms, we can use the Avogadro's number which is equal to 6.022 × 1023.
Avogadro's number is useful in determining the number of particles in a given quantity of substance.
The molecular weight of P2O5 is 141.94 g/mol.
From the formula, we can see that there are 2 phosphorus atoms present in one molecule of P2O5.
Using Avogadro's number, the number of moles of phosphorus atoms is given by: 1.00 × 10^6 phosphorus atoms/ 6.022 × 10^23 phosphorus atoms per mole= 0.001660 moles of phosphorus atoms
In one molecule of P2O5, there are 2 phosphorus atoms.
Therefore, the number of moles of P2O5 is given by: (0.001660 moles of phosphorus atoms) / 2= 0.000830 mol of P2O5
Now, we can use the molecular weight of P2O5 to calculate the mass of P2O5 in grams:
Mass = number of moles × molecular weight
= 0.000830 mol × 141.94 g/mol
= 0.118 g
Therefore, the mass of diphosphorus pentoxide (P2O5) that contains a million (1.00*10^6) phosphorus atoms is 0.118 g.
For more such questions on phosphorus, click on:
https://brainly.com/question/1357744
#SPJ11
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
catastrophic releases of hazardous chemicals must be investigated within
Answers
Catastrophic releases of hazardous chemicals must be investigated within the framework of appropriate regulatory and legal requirements. The specific jurisdiction and applicable regulations may vary depending on the country or region. However, some common frameworks for investigating such incidents include:
1. Occupational Safety and Health Administration (OSHA): In the United States, OSHA is responsible for ensuring safe and healthy working conditions. They investigate workplace incidents, including catastrophic releases of hazardous chemicals, to determine the cause and identify any violations of safety regulations.
2. Environmental Protection Agency (EPA): The EPA oversees environmental regulations and may investigate catastrophic chemical releases that pose risks to the environment and public health. They enforce laws such as the Clean Air Act and the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA).
3. Chemical Safety Board (CSB): The CSB is an independent federal agency in the United States that investigates chemical accidents and releases. Their focus is on determining the root causes of incidents, making recommendations to prevent future occurrences, and improving the overall safety of the chemical industry.
4. National or regional regulatory bodies: Other countries have their own regulatory agencies responsible for investigating hazardous chemical releases. For example, the Health and Safety Executive (HSE) in the United Kingdom and the National Institute for Occupational Safety and Health (NIOSH) in the United States conduct investigations related to workplace safety and health.
5. Industry-specific regulations: Certain industries may have specific regulations and oversight bodies dedicated to investigating incidents within their sector. For example, the Pipeline and Hazardous Materials Safety Administration (PHMSA) in the United States investigates incidents related to the transportation of hazardous materials.
It's important to note that investigations into catastrophic releases of hazardous chemicals often involve multiple agencies working together to assess the causes, impacts, and potential violations. These investigations aim to determine the root causes, identify any safety or regulatory failures, and make recommendations to prevent similar incidents in the future.
To know more about OSHA refer here
https://brainly.com/question/9178716#
#SPJ11
After extraction of the benzoic acid into the aqueous solution as its anionic salt, the ether solution was then washed with water two further times. What do you think was the purpose of these two wash steps?.
Answers
To remove any remaining unreacted inorganic component (from the added separating reagent) in the ether solution, two additional washes were performed after extracting benzoic acid into the aqueous solution as its anionic salt.
Benzoic acid must first be extracted from a mixture by adding NaOH, and then any excess NaOH must be removed in order to continue. Further, to get rid of the excess NaOH in the solution, it is washed with water twice or three times.
To reduce the amount of residue and other impurities in the layers, washing is done. The polar impurities, such as the leftover anionic salt of benzoic acid, tend to migrate to the water when washing, giving you a purer organic layer because ether is non-polar and water is polar.
Find more on benzoic acid at : brainly.com/question/19755712
#SPJ4
An alkyne with six carbon atoms per
molecule has relative molecular mass
of [C = 12, H = 1]
72,
82
84
86
Answers
Explanation:
C6H10
12(6) + 1(10)
= 72 + 10 = 82
The relative molecular mass of the alkyne with 6 carbon atoms is 82 gram/mole.
What is molecular mass?Molecular mass of a compound or a molecule is defined as the mass of the elements which are present in it.The molecular mass is considered to be a bulk quantity not a molecular quantity. It is often an average of the of the masses at many instances.
The molar mass and formula mass are used as synonym for the molar mass.It does not depend on the amount of substance which is present in the sample.It has units of gram/mole.
Molecular masses of an element are given as relative atomic masses while that of compounds is the sum of relative atomic masses which are present in the compound.Relative molecular masses are also calculated by the same method.
In case of an alkyne with formula C₆H₁₀ relative molecular mass is calculated as, 12×6 +1×10=82 g/mole.
Hence, the relative molecular mass of an alkyne with 6 carbon atoms is 82 g/mole.
Learn more about molecular mass,here:
https://brainly.com/question/18446366
#SPJ2
The widely-used raidoactive isotope of carbon 14c has an atomic number of 6, and a mass number of 14. how many neutrons does 14c have?
Answers
The widely used radioactive isotope of carbon 14 C has an atomic number of 6, and the mass number of 14. The number of the neutrons does 14C have is 8 neutrons.
The Atomic number = Number of the electrons = Number of protons = 6
Mass number is the sum of number of protons and neutrons in an atom.
The Mass number = Number of protons + Number of neutrons
14 = Number of protons + Number of neutrons
6 + Number of neutrons = 14
The Number of the neutrons = 8
Thus , there are the 8 neutrons, the 6 protons and the 6 electrons in carbon isotope.
To learn more about neutrons here
https://brainly.com/question/8639165
#SPJ4
CHEMISTRY HELP NEEDED
Why is critical mass important for a fission chain reaction?
- it keeps the neutrons from escaping the sample
- it keeps neutrons from being absorbed by other isotopes
- it allow neutrons to e absorbed by other fissionable nuclei
- it provides enough fuel to make enough energy
Why is a moderator important for a fission chain reaction?
- it keeps the neutrons from escaping the sample
- it keeps neutrons from being absorbed by other isotopes
- it allow neutrons to e absorbed by other fissionable nuclei
- it provides enough fuel to make enough energy
Why is enrichment important for a fission chain reaction?
- it keeps the neutrons from escaping the sample
- it keeps neutrons from being absorbed by other isotopes
- it allow neutrons to e absorbed by other fissionable nuclei
- it provides enough fuel to make enough energy
Answers
1. We can see here that critical mass is important for a fission chain reaction because: C. It allow neutrons to be absorbed by other fissionable nuclei.
What is fission chain reaction?Fission chain reaction is a self-sustaining reaction in which the splitting of atomic nuclei of a particular material, such as uranium or plutonium, releases a large amount of energy in the form of heat and radiation.
2. A moderator is important for a fission chain reaction because: A. it keeps the neutrons from escaping the sample.
3. Enrichment is important for a fission chain reaction because: D. it provides enough fuel to make enough energy.
A moderator is important for a fission chain reaction because it slows down the fast-moving neutrons, making them more likely to be absorbed by other fissionable nuclei and sustain the chain reaction. Without a moderator, the neutrons would move too quickly to be efficiently absorbed.
Learn more about fission chain reaction on https://brainly.com/question/24892298
#SPJ1
what is the molarity of a solution having 1.4 mol of sodium chloride, nacl, and a volume of 525 ml?
Answers
0.002M is the molarity of a solution having 1.4 mol of sodium chloride, nacl, and a volume of 525 ml
What does molarity mean exactly?
The number of moles of dissolved solute per litre of solution is the definition of molarity, a unit of concentration. Molarity is defined as the number of millimoles per millilitre of solution when the number of moles and the volume are divided by 1000.
A chemical species' concentration in a solution, specifically the amount of a solute per unit volume of solution, is measured by its molar concentration. The number of moles per litre, denoted by the unit sign mol/L or mol/dm3 in SI units, is the most often used unit denoting molarity in chemistry.
Molarity is no of moles/volume
1.4/525 i.e. 0.002M
To learn more about molarity use:
https://brainly.com/question/14469428
#SPJ4
23.54 for each of the following reactions, draw the complete mechanism and the major organic product(s). (a) h2n hno3 ? (b) h2n hno3 ? h2so4 acetic acid
Answers
(a) Mechanism: HNO3 acts as a strong acid, dissociating into H+ and NO3-. H+ protonates the amino group (H2N), forming NH3+. Nitration occurs by electrophilic aromatic substitution. NO3- acts as the electrophile, attacking the benzene ring.
The amino group donates a lone pair of electrons to the benzene ring, stabilizing the positive charge formed on the ring.
A proton (H+) transfers from NH3+ to the nitrogen atom, restoring aromaticity.
Water (H2O) deprotonates the OH group, resulting in the formation of the final product.
Major product: Nitrobenzene (C6H5NO2) (b) Mechanism:
HNO3 acts as a strong acid, dissociating into H+ and NO3-.
H+ protonates the amino group (H2N), forming NH3+.
Nitration occurs by electrophilic aromatic substitution. NO3- acts as the electrophile, attacking the benzene ring.
The amino group donates a lone pair of electrons to the benzene ring, stabilizing the positive charge formed on the ring.
A proton (H+) transfers from NH3+ to the nitrogen atom, restoring aromaticity.
H2SO4 acts as a catalyst, promoting the addition of an acetyl group (CH3CO) to the amino group.
Water (H2O) deprotonates the OH group, resulting in the formation of the final product.
Major product: N-acetylaniline (C6H5NHCOCH3)
learn more about acid here:
https://brainly.com/question/29796621
#SPJ11
Convert the atmospheric pressure found in this lab from mm Hg to atm. Round to three sig figs.
760 mm Hg x 1 atm/ 760 mmHg= 1.00664474
Answers
The atmospheric pressure in the lab in atm is 1 atm.
Given atmospheric pressure in mm Hg is 760.
We need to convert this pressure from mm Hg to atm.
Atmospheric pressure can be measured in different units like mmHg, atm, Pa, and torr.
Here, we are going to convert the pressure from mmHg to atm using the following conversion factor.
1 atm = 760 mmHg
To convert from mmHg to atm, we divide the value in mmHg by 760.
760 mmHg = 760/760 atm = 1 atm
Therefore, the atmospheric pressure in atm is 1 atm.
We can also use the conversion factor to convert the pressure from mmHg to atm as follows:
760 mmHg x (1 atm/760 mmHg) = 1 atm
Hence, the atmospheric pressure found in the lab in atm is 1 atm.
The given conversion has been executed below.
760 mmHg × (1 atm / 760 mmHg) = 1 atm
Therefore, the atmospheric pressure in the lab in atm is 1 atm.
To know more about atmospheric pressure, visit:
https://brainly.com/question/28310375
#SPJ11