Answers
Answer:
The answer is Rubidium
Explanation:
The number of protons is unique for each element. It is also identified as an element’s atomic number. Look for an element with the number of protons as the atomic number to find the unidentified element.
Answer:
Hello again, I think the option will be the third one Rubidium im highly sure because the periodic table goes by the number of protons which in your question is 37. So yeah it will be rubidium.
Explanation:
Look for 37 protons in the periodic table.
Related Questions
a rigid cylinder at that temperature contains 0.127 atm of hydrogen, 0.134 atm of iodine, and 1.055 atm of hydrogen iodide. is the system at equilibrium?
Answers
The measured Kc is different from the calculated Kc, the system is not at equilibrium. There is either too much hydrogen iodide and/or too little hydrogen and/or iodine in the cylinder.
To determine whether the system is at equilibrium, we can use the equilibrium constant expression for the reaction;
H₂(g) + I₂(g) ⇌ 2HI(g)
The equilibrium constant expression for this reaction is;
Kc = [HI]² / [H₂] [I₂]
We are given the partial pressures of each component in the cylinder, so we can calculate the concentrations using the ideal gas law.
[P] = n/V = (m/M) / V
where P is the partial pressure, n is the number of moles, V is the volume, m is the mass, M is the molar mass, and the square brackets denote concentration.
For hydrogen (H₂)
[P] = n/V = (m/M) / V
[m/M] = [P] x V
[m/M] = 0.127 atm x V / R x T
For iodine (I₂)
[P] = n/V = (m/M) / V
[m/M] = [P] x V
[m/M] = 0.134 atm x V / R x T
For hydrogen iodide (HI)
[P] = n/V = (m/M) / V
[m/M] = [P] x V
[m/M] = 1.055 atm x V / R x T
Switching these expressions into the equilibrium constant expression, we get;
Kc = ([HI]/V)² / ([H₂]/V) x ([I2]/V)
Kc = ([HI]² / V²) / ([H₂] x [I2] / V²)
Put in the values we get:
Kc = [(1.055 atm / V)² / (0.127 atm / V) x (0.134 atm / V)]
Kc = 8.37 atm² / V²
If the system is at equilibrium, the measured concentrations should give the same value for Kc as calculated above. If the measured Kc is different, then the system is not at equilibrium.
Therefore, we can calculate the measured Kc as;
Kc = ([HI]² / V²) / ([H₂] x [I2] / V²)
Kc = [(1.055 atm / V)² / (0.127 atm / V) x (0.134 atm / V)]
Kc = 8.87 atm² / V²
To know more about equilibrium constant here
https://brainly.com/question/10038290
#SPJ4
I'll give brianliest if correct .

Answers
2.60x1023 molecules of hexane (C6H14) and 7.00x1023 molecules of O2 are available for a combustion reaction. Theoretically, how many molecules of water can be produced?
Answers
The first step is to write the combustion reaction of hexane:
\(\begin{gathered} 2C_6H_{14}+19O_2\rightarrow12CO_2+14H_2O \\ \end{gathered}\)Now, we can use Avogadro's Number to find the amount of moles of hexane and O2 that are reacting:
\(\begin{gathered} 2.60\times10^{23}molecules\cdot\frac{1mol}{6.023\times10^{23}molecules}=0.43molC_6H_{14} \\ 7.00\times10^{23}molecules\cdot\frac{1mol}{6.023\times10^{23}molecules}=1.16molO_2 \end{gathered}\)Using the stated reaction we can determine the amount of moles of O2 that react with 0.43 moles of hexane:
\(0.43moles\cdot\frac{19molesO_2}{2molesC_6H_{14}}=4.085molesO_2\)From this we can conclude that the limiting reactant is the O2, which means that we have to use the amount of O2 to make the calculations. Use this amount and the stoichiometric ratio of O2 and H2O to find the amount of water produced from 1.16moles of oxygen:
\(1.16molO_2\cdot\frac{14molH_2O}{19molO_2}=0.85molesH_2O\)Now, we can use Avogadro's number to convert the amount of moles produced to molecules:
\(0.85molesH_2O\cdot\frac{6.023\times10^{23}moleculesH_2O}{1molesH_2O}=5.12\times10^{23}moleculesH_2O\)It means that 5.12x10^23 molecules of water are produced.
A company charges $5.00 to rent a paddle boat for the first hour, plus $2.00 for each additional hour.
Answers
When 1.0 mole of Zno(s) decomposes the standard enthalpy change, AH, is 350 kJ/mol. What does this tell you about the decomposition of ZnO(s)? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer a The decomposition of ZnO is endothermic, b The heat of reaction for the decomposition of ZnO is exothermic c The formation of ZnO does not require energy d The heat of the calorimeter for the decomposition of ZnO is endothermic.
Answers
When 1.0 mole of ZnO(s) decomposes, the standard enthalpy change, ΔH, is 350 kJ/mol. This tells us that the decomposition of ZnO is endothermic (A).
What is the standard enthalpy change?The standard enthalpy change, ΔH, refers to the energy change that occurs when one mole of a substance reacts or undergoes a physical change under standard conditions (25°C and 1 atm). This enthalpy change is often associated with reactions or physical changes that are exothermic or endothermic.
What is the meaning of endothermic reaction?An endothermic reaction is a type of chemical reaction in which energy is absorbed from the surroundings in the form of heat. This results in an increase in the internal energy of the system as the reactants are converted to products, and the surroundings become colder. In contrast, exothermic reactions release heat to the surroundings.
Hence, the correct answer is Option A.
Learn more about endothermic reaction here: https://brainly.com/question/30133682
#SPJ11
Calculate the volume of water which was produced when 1120 cm cubics of oxygen at s.t.p was liberated during the decomposition of hydrogen peroxide
Answers
Volume of H₂O produced = (1.8 g) × (1 cm³/g) = 1.8 cm³
Simplifying :
Molar volume of O₂ at STP = 22400 cm³/mol
No. of moles of O₂ liberated = (1120 cm³) / (22400 cm³/mol) = 0.05 mol
According to the given equation, mole ratio H₂O : O₂ = 2 : 1
No. of moles of H₂O produced = (0.05 mol) × 2 = 0.1 mol
Molar mass of H₂O = (1×2 + 16) g/mol = 18 g/mol
Mass of H₂O produced = (0.1 mol) × (18 g/mol) = 1.8 g
Volume of H₂O produced = (1.8 g) × (1 cm³/g) = 1.8 cm³
What happens in the decomposition of hydrogen peroxide?
The decomposition of hydrogen peroxide can be summarized by the chemical equation: which states that two molecules of hydrogen peroxide break down to form two molecules of water and one molecule of oxygen gas, along with heat energy.
Why does hydrogen peroxide decompose quickly?
In many living organisms hydrogen peroxide is a product of metabolism that must be broken down, since in appreciable concentrations it is toxic. The rate of decomposition is increased by the intra-cellular enzyme catalase.
At what temperature does hydrogen peroxide decompose?
Properties. The boiling point of H 2O 2 has been extrapolated as being 150.2 °C (302.4 °F), approximately 50 °C (90 °F) higher than water. In practice, hydrogen peroxide will undergo potentially explosive thermal decomposition if heated to this temperature
Learn more about decomposition of hydrogen peroxide:
brainly.com/question/20418092
#SPJ4
Wade thinks it would be really cool to become a radiologist. Which two skills are important for him to have in order to excel in this career?
Answers
Wade will need the following two abilities to succeed in the field of radiology:
1. Interpersonal abilities to communicate with patients and make them feel at ease.
2. Technical expertise required to operate machinery and equipment
What is a radiologist's role?A radiologist is a specialty physician who employs imaging techniques to diagnose and treat human illness or damage, including x-ray, MRI, CT, ultrasound, and angiography. Radiologists must complete extensive training and testing in order to receive accreditation from the appropriate governing bodies.
Do radiologists practice surgery?A subspecialty of radiology is called interventional radiology (IR). Through tiny incisions in your body, doctors execute minimally invasive surgical operations while also interpreting your medical images in interventional radiology.
To know more about Radiologists visit:
https://brainly.com/question/14617595
#SPJ9
Suppose you were given a substance and asked
to determine whether or not it was a plasma. Write
the characteristics you would look for to identify
the substance.
Answers
Answer:Particles in plasmas collide more often.
Plasma particles have high kinetic energy (they move quickly).
Plasma particles are far apart.
The ionized particles have no fixed volume
Explanation:
Plasma is the fourth form of matter which has freely moving electrons in it. The substance can be identified as plasma if its particles collide often and are far apart.
What is plasma?Plasma is a type of matter other than solids, liquids, and gas. They have similar properties to that of gas. Plasma particle collides are often like gases as they move freely in space. Like gases that have no definite shape and volume.
The plasma particles have ionic charges, positive and negative resulting in high kinetic energy. This property allows them to show electromagnetism and electrical conductivity.
The ionized particles are due to the high temperature that allows them to have the property of electrical conductivity and compression.
Therefore, the particles of plasma are ionized and have high kinetic energy.
Learn more about plasma here:
https://brainly.com/question/10133920
#SPJ6
Which of the following is a characteristic of mixture
Answers
Answer:
One of the most notable characteristics of a mixture is that it does not have a fixed composition. Water, a compound, always has 11.2 percent hydrogen and 88.8 percent oxygen by weight. Brass is a mixture because it has varying amounts of copper and zinc. One type of brass may contain as much as 45 percent zinc, while another type may contain around 10 percent zinc. Mixtures may also have changing properties depending on their composition. The boiling point of a mixture of alcohol and water varies based on how much alcohol is in the mixture.
Hope this helps :)
A group of scientists is testing a new chemical to determine if it will act as a sunscreen and prevent sun burn. The same amount of lotion is applied to each person in the same size area and the test subject's arm is exposed to direct sunlight.The amount of redness is measured every 5 mins for a total of 30 mins. For each person tested, 3 different lotions are applied to the test subject's arm:
1. a lotion with the new sunscreen chemical called Sun X.
2. a lotion with a different active sunscreen chemical that has been sucessfully used for many years called Sun Kissed.
3. a lotion that has all the same ingredients as the sunscreens except it has no nactive unscreen chemical.
1. What question is being asked in the experiment above?
2. What hypothesis is being tested in the experiment described above?
a. Is the hypothesis testable and fausible? Explain Why
3.What is the prediction based on the hypothesis?
4.What is the independent variable in this experiment?Explain why
5. What is the depende\nt variable in this experiment?Explain why
6. Which group of patients is the experimental group? Expalin why
7. which group of patients is the negative control? Explain why
8.Which group of patients is the positive control?Explain why
Answers
1. The question being asked in the experiment above is, "Will the lotion with the new chemical act as a sunscreen and prevent sunburn?"
2. The hypothesis that is being tested in the experiment described above is, "If the lotion with the new sunscreen chemical (Sun X) is applied, then it will act as a sunscreen and prevent sunburn more effectively than the lotion with the different active sunscreen chemical (Sun Kissed) and the lotion that has all the same ingredients except no active sunscreen chemical.
Yes, the hypothesis is testable and falsifiable. It is testable because it can be tested through the experiment described above. It is falsifiable because it can be proven false if the lotion with the new sunscreen chemical (Sun X) does not prevent sunburn more effectively than the other two lotions.
3. The prediction based on the hypothesis is that the lotion with the new sunscreen chemical (Sun X) will act as a sunscreen and prevent sunburn more effectively than the lotion with the different active sunscreen chemical (Sun Kissed) and the lotion that has all the same ingredients except no active sunscreen chemical.
4. The independent variable in this experiment is the type of lotion being applied to the test subject's arm. This is because the researcher can manipulate the type of lotion being applied.
5. The dependent variable in this experiment is the amount of redness measured on the test subject's arm. This is because the amount of redness depends on the type of lotion being applied.
6. The experimental group is the group of patients that are given the lotion with the new sunscreen chemical (Sun X). This is because this group is being tested to determine if the new chemical acts as a sunscreen and prevents sunburn.
7. The negative control group is the group of patients that are given the lotion that has all the same ingredients except no active sunscreen chemical. This is because this group is being tested to ensure that any differences observed between the other two groups are due to the presence or absence of the active sunscreen chemical, rather than any other factor.
8. The positive control group is the group of patients that are given the lotion with the different active sunscreen chemical (Sun Kissed). This is because this group is being tested to ensure that the experiment is able to detect differences in the effectiveness of different sunscreens.
To know more about Sunscreens visit:
https://brainly.com/question/20448560
#SPJ11
What’s the answer to this?
Answers
What dose the wave carry
Answers
Answer:
Waves carry energy from one place to another.
Because waves carry energy,some waves are used for communication,eg radio and television waves and mobile telephone signals.
Explanation:
i hope it helps
that's my answer
correct me if im wrong
#carryonlearningComplete and balance the following half-reaction in acidic solution.
Answers
Answer: Heyo Kenji Here! Here's your answer- N2(g) → NH4+(aq)
Explanation: Hope this helps!
Have a nice day!
- Kenji ^^
Please help me with number 12

Answers
Answer:
organisms in rain water and the number of organisms in pond water because the dependant variable never changes
Explanation:
because the dependant variable never changes
How many moles are in 20 grams of O₂ gas?
Answers
In NH2Br, there are ___________ number of total bonds. Of those bonds, __________ are polar bonds, and __________ are non-polar bonds
Answers
In NH2Br, there are a total of 6 bonds. To determine the number of polar and non-polar bonds, we need to examine the electronegativity difference between the atoms involved in each bond.
In NH2Br, the central atom is nitrogen (N), and it is bonded to two hydrogen atoms (H) and one bromine atom (Br).
The N-H bonds are polar because nitrogen is more electronegative than hydrogen, creating a partial negative charge on nitrogen and a partial positive charge on hydrogen.
The N-Br bond is also polar because nitrogen is more electronegative than bromine, resulting in a partial negative charge on nitrogen and a partial positive charge on bromine.
Therefore, in NH2Br, there are 3 polar bonds (N-H, N-H, N-Br) and 3 non-polar bonds (H-H, H-H, Br-H).
To summarize:
Total bonds: 6
Polar bonds: 3 (N-H, N-H, N-Br)
Non-polar bonds: 3 (H-H, H-H, Br-H)
It's important to note that the polarity of a bond is determined by the electronegativity difference between the atoms involved. If the electronegativity difference is significant, the bond is considered polar; otherwise, it is considered non-polar.
To know more about bond click this link -
brainly.com/question/30508122
#SPJ11
Which mass and atomic number is correct for the following isotope: carbon-14
explain your answer

Answers
Answer:
Explanation:
Isotope notation is of the form
A
Z
X
where
Z
is the atomic number (number of protons),
A
is the mass number (sum of protons and neutrons), and X is the element.
In Carbon- 14 , 14 is the mass number (sum of protons and neutrons). This is our A .
Carbon has the atomic symbol C , and it has 6 protons. This is the atomic number, or A . Thus, the isotope notation for Carbon- 14 is 14 6 C
Hope this helps!
Explanation:
2. What mass of sodium chloride is produced when chlorine gas reacts with 2.29 grams of sodium iodide? The unbalanced equation is given below: Nal (s) + Cl, (g) → NaCl (s) + 1, (g)
Answers
Answer:
0.88g
Explanation:
The reaction equation:
2NaI + Cl₂ → 2NaCl + I₂
Given parameters:
Mass of Sodium iodide = 2.29g
Unknown:
Mass of NaCl = ?
Solution:
To solve this problem, we work from the known to the unknown.
First find the number of NaI from the mass given;
Number of moles = \(\frac{mass}{molar mass}\)
Molar mass of NaI = 23 + 126.9 = 149.9g/mol
Now insert the parameters and solve;
Number of moles = \(\frac{2.29}{149.9}\) = 0.015mol
So;
From the balanced reaction equation;
2 moles of NaI produced 2 moles of NaCl
0.015mole of NaI will produce 0.015mole of NaCl
Therefore;
Mass = number of moles x molar mass
Molar mass of NaCl = 23 + 35.5 = 58.5g/mol
Now;
Mass of NaCl = 0.015 x 58.5 = 0.88g
4 NH3 + 7 O2 → 4 NO2 + 6 H2O What is the mole ratio between oxygen and nitrogen dioxide? 7 moles to 6 moles 4 moles to 6 moles 7 moles to 4 moles 4 moles to 4 moles
Answers
Answer:
7:4
Explanation:
O2 : NO2
7 : 4
hope this helps :)
PLS HELP ASAP
salt water is a pure substance
true or false?
Answers
Answer:
false
Explanation:
salt water cannot be classified as a substance because it's composition can vary
naturally occuring element x exists in three isotopic forms: x-28 (27.343 amu, 67.14% abundance), x-29 (28.889 amu, 10.50% abundance), and x-32 (31.993 amu, 22.36% abundance). calculate the average atomic weight of x. please enter your answer to 4 significant figures.
Answers
The naturally occurring the element x exists in three isotopic forms. The average atomic weight of x 28.544 amu.
Given that :
x- 28, Abundance % = 67.14 % = 0.6714
The atomic mass = 27.343 amu
x-29, Abundance % = 10.50 % = 0.1050
The atomic mass = 28.889 amu
x- 32, Abundance % = 22.36 % = 0.2236
The atomic mass = 31.993 amu
The average atomic weight = ( 27.343 × 0.6714 ) + ( 28.889 × 0.1050) +
( 31.993 × 0.2236)
= 18.358 + 3.0333 + 7.153
= 28.544 amu
Thus, the average atomic weight of naturally occurring element x exists in three isotopic forms 28.544 amu.
To learn more about average atomic weight here
https://brainly.com/question/28013988
#SPJ4
Why is elemental zinc considered a pure substance?
Select one:
It is a metal.
It is a mixture of different atoms.
All elements are considered pure substances.
It must be created in a lab.
Answers
Answer: C... Because Zinc is made of only one type of atoms.
Explanation: All elements are made of one type of atom. Pure gold is made out of only gold atoms, silver out of only silver atoms, etc.
When describing the electrons in an orbital, we use arrows pointing upward and downward to indicate what property?
(A.) electron configuration
(B.) electron orbitals
(C.) electron charge
(D.) electron spin
Answers
Answer:
D.) electron spin
Each sublevel is labeled by its principal energy level and sublevel. Electrons are indicated by arrows inside of the circles. An arrow pointing upwards indicates one spin direction, while a downward pointing arrow indicates the other direction
Logan demonstrates to the class how mass must be conserved in every chemical reaction.
He measures the mass of hydrochloric acid and a magnesium strip separately. He then places the magnesium strip into the acid and bubbles form as the magnesium
seems to disappear. The combined mass afterward is less than the original.
Hydrochloric Acid + Magnesium
How could Logan explain this lower mass?
Answers
Answer:
Logan could explain the lower mass by explaining that the reaction between hydrochloric acid and magnesium produces hydrogen gas, which escapes and therefore is not included in the final combined mass measurement. The reaction is:HCl + Mg → MgCl2 + H2Since the hydrogen gas has mass, its escape from the reaction vessel explains the decrease in the combined mass of the reactants after the reaction.
please answer these about Charles law


Answers
Answer:
1. V2.
2. 299K.
3. 451K
4. 0.25 x 451 = V2 x 299
Explanation:
1. The data obtained from the question include:
Initial volume (V1) = 0.25mL
Initial temperature (T1) = 26°C
Final temperature (T2) = 178°C
Final volume (V2) =.?
2. Conversion from celsius to Kelvin temperature.
T(K) = T (°C) + 273
Initial temperature (T1) = 26°C
Initial temperature (T1) = 26°C + 273 = 299K
3. Conversion from celsius to Kelvin temperature.
T(K) = T (°C) + 273
Final temperature (T2) = 178°C
Final temperature (T1) = 178°C + 273 = 451K
4. Initial volume (V1) = 0.25mL
Initial temperature (T1) = 299K
Final temperature (T2) = 451K
Final volume (V2) =.?
V1 x T2 = V2 x T1
0.25 x 451 = V2 x 299
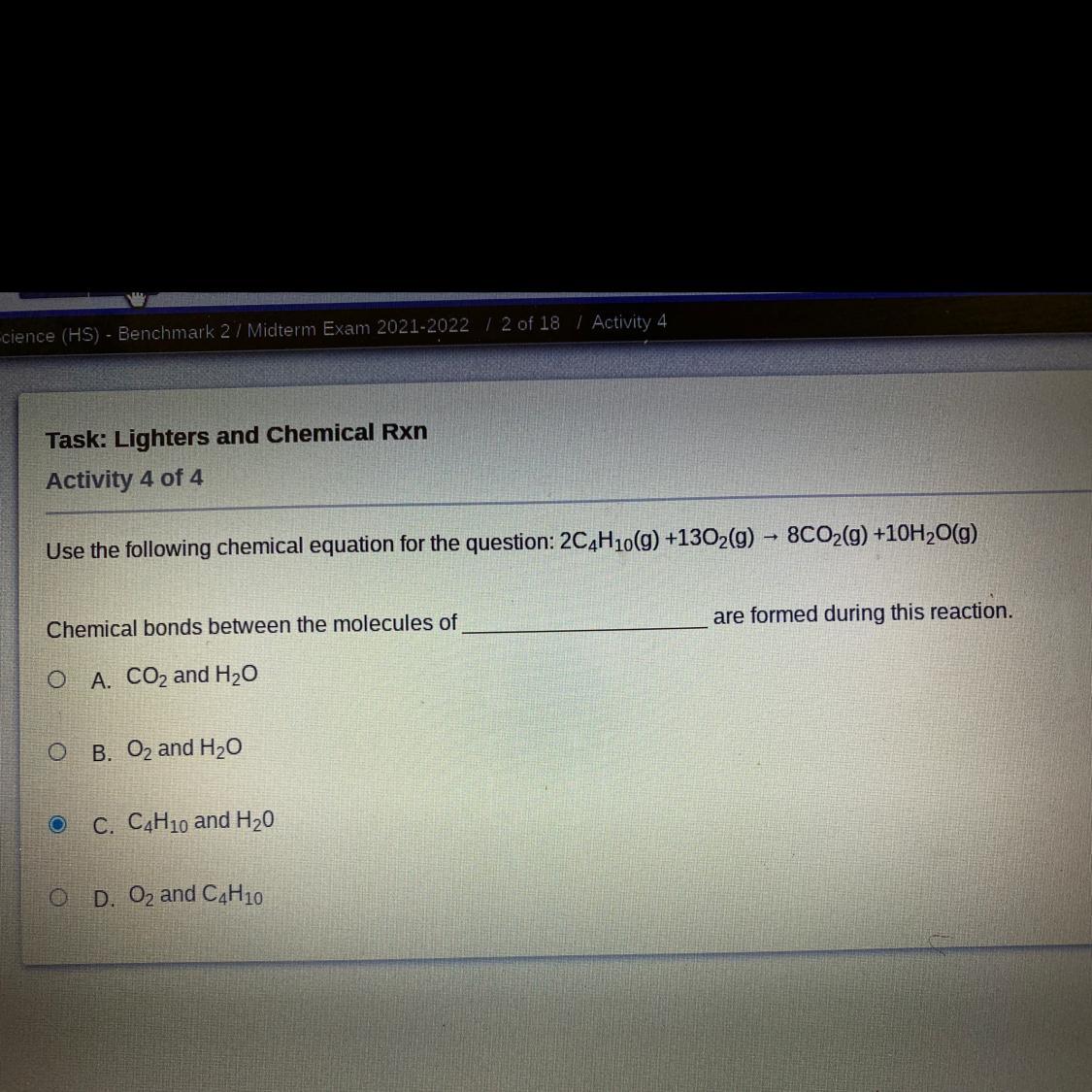
1: _C4H10+_O2=_CO2+_H2O
Answers
Answer:
2C4H10 + 13O2 → 8CO2 + 10H2O
Hope this helps brainleist??
Explanation:
HELP THIS IS FOR CHEM!! Which of the following are contact forces? Choose three.
Tension, magnetic, electric, gravity, normal,friction,weight.
Answers
Answer:
Tension, friction, and normal
Explanation:
Cassiterite is an ore of a metal tin. What is an ore?
Answers
Answer:
Cassiterite is an ore of Sn.It is also called tinstone and has molecular formula of SnO
2 . It is heavy, metallic, hard tin dioxide that is the major ore of tin. It is colourless when pure, but brown or black when iron impurities are present.
rank the ions in each set in order of increasing size. a. li , k , na b. se2– , rb , br – c. o2– , f – , n3–
Answers
The correct order of increasing size is in each set is: Li⁺ < Na⁺ < K⁺, Br⁻ < Se²⁻ < Rb⁺, and N³⁻ < O²⁻ < F⁻.
a. In order of increasing size, the ions in set a are: Li, Na, K. This is because they all have the same charge (+1), but as you move down the periodic table, the atomic radius increases.
b. In order of increasing size, the ions in set b are: Br-, Se2-, Rb. This is because Br- and Se2- have the same charge (-1), but as you move down the periodic table, the atomic radius increases. Rb has a larger atomic radius than Se, which gives it a larger ionic radius.
c. In order of increasing size, the ions in set c are: N3-, O2-, F-. This is because they all have the same charge (-1), but as you move across the periodic table, the atomic radius decreases. F- has the smallest atomic radius, which gives it the smallest ionic radius.
Know more about Atomic Radius here:
https://brainly.com/question/14086621
#SPJ11
What point does the ammonium ions act as a buffer solution? Please this is driving me insane.

Answers
Answer:
Explanation:The buffer is a mixture of ammonia (NH3) and ammonium (NH4+). ... Since ammonia (NH3) is a weak base, it will have a pH above 7 and since ammonium (NH4+) is a weak acid, it will have a pH below 7. Ammonia (NH3) is a weak base and ammonium (NH4+) is a weak acid. Ammonia (NH3) is the conjugate base of ammonium (NH4+).
