Answers
Answer:
its b
Explanation:
Answer: the first box
Explanation:
Related Questions
Read and find the mechanism by which the following enzymes (more than two substrates) work?! 1. Glyceraldehyde-3-phosphate dehydrogenase D-glyceraldehyde-3-phosphate + NAD+ + P; 3-phospho-D-glycerol phosphate + NADPH + 2. Glutamate dehydrogenase 2-ketoglutarate + NH4+ + NAD(P)H L-glutamate + NAD(P)* + H2O 3. Isocitrate dehydrogenase 2-ketoglutarate + CO2 + NADH isocitrate + NAD+
Answers
These are simplified explanations of the mechanisms involved in these enzyme-catalyzed reactions, highlighting the key steps and substrate interactions.
Glyceraldehyde-3-phosphate dehydrogenase:
The mechanism of Glyceraldehyde-3-phosphate dehydrogenase involves multiple substrates. Here's a step-by-step explanation:
D-glyceraldehyde-3-phosphate, NAD+, and P bind to the active site of the enzyme.
The enzyme catalyzes the oxidation of D-glyceraldehyde-3-phosphate by transferring a hydride ion (H-) from D-glyceraldehyde-3-phosphate to NAD+, forming NADH.
The P (inorganic phosphate) binds to the carbonyl group of the oxidized D-glyceraldehyde-3-phosphate, resulting in the formation of 3-phospho-D-glycerol phosphate.
NADH and 3-phospho-D-glycerol phosphate are released from the active site of the enzyme.
Glutamate dehydrogenase:
The mechanism of Glutamate dehydrogenase also involves multiple substrates. Here's a step-by-step explanation:
2-ketoglutarate, NH4+, and NAD(P)H bind to the active site of the enzyme.
The enzyme catalyzes the oxidative deamination of 2-ketoglutarate by transferring an amine group (NH3) from 2-ketoglutarate to NAD(P)H, forming NAD(P)+ and L-glutamate.
H2O is added to the amine group of the intermediate L-glutamate, resulting in the formation of L-glutamate as the final product.
NAD(P)+ and H2O are released from the active site of the enzyme.
Isocitrate dehydrogenase:
The mechanism of Isocitrate dehydrogenase also involves multiple substrates. Here's a step-by-step explanation:
2-ketoglutarate, CO2, and NADH bind to the active site of the enzyme.
The enzyme catalyzes the oxidative decarboxylation of 2-ketoglutarate by removing a carboxyl group (CO2) from 2-ketoglutarate, resulting in the formation of isocitrate and NAD+.
NAD+ is reduced to NADH during this step.
Isocitrate is converted into an intermediate that undergoes isomerization, forming α-ketoglutarate.
NADH and α-ketoglutarate are released from the active site of the enzyme.
These are simplified explanations of the mechanisms involved in these enzyme-catalyzed reactions, highlighting the key steps and substrate interactions. The actual mechanisms may involve additional intermediate steps and cofactors.
Learn more about catalyzed with the given link ,
https://brainly.com/question/21598276
#SPJ11
I NEED HELP ASAP!!!!!!!!!!!
WITHIN THE HOUR
Thanks

Answers
Li = 6
Be =9
C= 12
O=15
Answer:
C
Explanation:
Actually, arranged to the size of smallest to least atom radius size = C
Atomic sizes generally get smaller as you go from L to R across a row of the periodic table.
if you have ever looked around you and thought about the way things work, you have made a(n) ____________________.
Answers
Answer:
observation
Explanation:
3. Which of the following represents a covalent compound?
a. 02
b. CO2
C. Cl2
d. Naci
Answers
For the Question "Which of the following represents a covalent compound?" we have
CO_2Option BFrom the Question we are told
'3. c
Generally
a covalent compound is a kind of bond in which valence electrons are shared b/w the party atoms forming the bond
Therefore
We see covalent compound in CO_2 as they match perfectly the phenomenon that is covalent bonding
Therefore
For the Question "Which of the following represents a covalent compound?" we have
CO_2Option BFor more information on this visit
https://brainly.com/question/14418346?referrer=searchResults
Charli is boiling a pot of water as she prepares dinner. The temperature at which the water boils can be explained by the external
pressure and
A
the amount of heat that is added to the pot of water.
B. the volume of the sample of water being heated.
C. the rate at which heat is added to the pot of water.
D
the strength of attractive forces among the water molecules.
PLEASE ANSWER QUICKLY THIS IS DUE IN 5 MINS!!
Answers
Answer:
ya tu sabes cómo se los aseguro
Based on patterns in the periodic table, which list'shows the acids in order from strongest to weakest?
(1 point)
OHCI, H2S, PH3
OHCI, PH3, H2S
OH,S, PH3, HCI
PH3, H,S, HCI
Answers
Answer:
it is A.HCI, H2S, PH3
Explanation:
I took the test
Which of these chemical equations describes a precipitation reaction?Question options:A. 2H2(g) + O2(g) → 2H2O(l)B. BaBr2(aq) + H2SO4(aq) → BaSO4(s) + 2HBr(aq)C. 2KNO3(s) → 2KNO2(s) + O2(g)D 2KI(aq) + Cl2(g) → 2KCl(aq) + I2(s)
Answers
The chemical equation that describes a precipitation reaction is option B:
BaBr₂(aq) + H₂SO₄(aq) → BaSO₄(s) + 2HBr(aq)
In this equation, when barium bromide (BaBr₂) reacts with sulfuric acid (H₂SO₄), it forms barium sulfate (BaSO₄) as a solid precipitate, which is indicated by the "(s)" state symbol. The other product formed is hydrobromic acid (HBr) in the aqueous state, as indicated by the "(aq)" state symbol.
Precipitation reactions occur when two aqueous solutions react to form an insoluble solid product, known as a precipitate. In this case, the barium sulfate precipitates out of the solution as a solid, indicating a precipitation reaction.
To know more about Precipitation reaction:
https://brainly.com/question/30696406
#SPJ4
can someone help me

Answers
Answer:
it is alredy balanced
Explanation:
CaCO3 -------> CaO + CO2
In the reactant side In the product side
Ca = 1 atom Ca = 1 atom
C = 1 atom C = 1 atom
O = 3 atom O = 1+2 = 3 atom
so there is no need to balance it cause it is already balanced.
1
The faster air molecules move, the greater pressure they apply on the walls of their container.
In which of the following situations will the air pressure inside the container be the greatest?
(1 Point)
O A. a frozen bottle of water
B. a chilled bottle of fruit tea
O C. a juice box at room temperature
O D. a sealed container of hot chocolate
Answers
Answer:
D
Explanation:
the higher temperature is, the faster air molecules move and the air will expand. Moreover, the container is sealed, which means the pressure laying on its internal surface is higher than any cases above
Do the ingredients changing into a cake in the oven a chemical reaction?
Answers
Answer:
When you bake a cake, the ingredients go through a chemical change. A chemical change occurs when the molecules that compose two or more substances are rearranged to form a new substance! When you start baking, you have a mixture of ingredients. The flour, egg, sugar,
Explanation:
What information can a mineralogist learn from testing the mass and volume of a mineral?
Answers
A mixture of 1-butanol (1) + water (2) forms an azeotrope where x," - 0.807 und T - 335.15 K. Assuming the following relations apply for the activity coefficients: In y - 1) In yn - A) Given: Prat = 8.703 kPa and Prat = 21.783 kPa (a) Derive an expression for G/RT as a function of A and xi (b) Determine the numerical value of the constant (c) Using modified Raoult's law, determine the pressure atx" -0.807 and T-335.15 K.
Answers
To derive an expression for G/RT as a function of A and xi, we start with the Gibbs-Duhem equation: Σxi d(ln γi) = 0.
Integrating this equation gives: ∫d(ln γi) = 0. Integrating again and using the relation ln γi = ln yi - ln xi, we have: ln yi - ln xi = A ln xi + B. Rearranging the equation, we get: ln yi = (A + 1) ln xi + B. Taking the exponential of both sides, we obtain: yi = Kxi^(A+1), where K = e^B. (b) To determine the numerical value of the constant K, we can use the given data. At x" = 0.807, the mole fraction of the more volatile component (water) is yn = 0.807. Substituting these values into the equation above, we have: 0.807 = K(0.807)^(A+1).
Simplifying, we get: K = 0.807^(1-A). (c) Using the modified Raoult's law, the pressure at x" = 0.807 and T = 335.15 K can be determined. The modified Raoult's law equation is: P = Σxi γi P^sat, where P^sat,i is the vapor pressure of component i. Assuming an ideal gas mixture, we can use the Antoine equation to estimate the vapor pressures. Solving the equation above for P and substituting the given mole fraction and activity coefficient (A = -0.807), we can calculate the pressure at x" = 0.807 and T = 335.15 K.
To learn more about Gibbs click here: brainly.com/question/13795204
#SPJ11
write the net-ionic reaction that would occur between nitric acid and any carbonate ions in solution.
Answers
The net ionic equation for the reaction between nitric acid (HNO3) and a carbonate ion (CO32-) in solution is:
2H+ + CO32- → CO2 + H2O
In this reaction, the H+ ions from the nitric acid react with the CO32- ions from the carbonate, producing carbon dioxide (CO2) and water (H2O). The nitrate (NO3-) ion does not participate in the reaction and is therefore not included in the net ionic equation.
To know more about nitric acid refer here:
https://brainly.com/question/29769012
#SPJ11
What is the mass of calcium oxide produced in the reaction?
Answers
Answer:
From the reaction stoichiometry, 2 moles of Ca produces 2 moles of CaO. Thus, the number of moles of Ca is the same as that of CaO. Therefore, n = 0.16892 mol for CaO. Hence, 9.47 grams of calcium oxide are produced.complete the structure of the monosaccharide present in dna. the sugar should be in its β-furanose form.
Answers
The monosaccharide present in DNA is β-D-2-deoxyribose.
1. Identify the monosaccharide present in DNA: The sugar in DNA is a deoxyribose, which is a modified form of ribose where one of the hydroxyl (OH) groups is replaced by a hydrogen (H) atom.
2. Specify the configuration: The configuration of this sugar is D, meaning the molecule is a right-handed isomer.
3. Determine the furanose form: Furanose refers to a 5-membered ring structure that includes four carbon atoms and one oxygen atom. In the β-furanose form, the anomeric carbon (C1) has the same orientation as the highest numbered chiral carbon (C5 in this case).
4. Assemble the structure: To complete the structure, draw a 5-membered ring with four carbons and one oxygen, with the hydroxyl groups and hydrogen atoms attached to the appropriate carbons. The β-D-2-deoxyribose structure will have the hydroxyl group on C1 facing upward, in the same direction as the -CH2OH group on C5.
learn more about monosaccharide
https://brainly.com/question/27976384
#SPJ11
Name two responsible uses of machines and two irre- sponsible uses.
Answers
Machines make our works easy and fast. Nowadays there are many modern small and bigger machines designed to meet particular requirements.
What are machines?Machines are apparatus with several parts works with the use of mechanical power. There are large number of types of machines which are designed for different purposes in every field, agriculture, medical field, research field etc.
There are many small machines such as screw gauges to large machines used in power generators and machines in factories and laboratories. Machines are working in a programmed way with in desired time and make us effortless.
Machines can be used in non-genuine and non -commercial purposes also. However, nowadays novel machines are designing to be employed to meet todays requirements.
Find more on machines:
https://brainly.com/question/2641843
#SPJ1
Which of the following correctly illustrates the conservation of mass for the reaction below? I choose B but I’m not sure if I’m correct!

Answers
Answer: A
\(Na(23\times4=92g);O2(16\times2=32g);Na2O(23+23+16)\times2=124\)Explanation: Based on the Law of conservation of mass the total mass of the reactants will equal to the total mass of the products. This happens as matter is not destroyed.
Chalk is a silicate carbonate evaporite sandstone QUESTION 33 a photosyntehtic creature with a silica shell can be a O coccolithophorid foraminifer diatom radiolarian QUESTION 34 recrystallization of chalk at the ocean bottom (not in metamorphic conditions) can give us O micrite chert marble quartzite
Answers
Diatoms are single-celled algae that have a silica (silicate) shell called a frustule.
Diatoms are photosynthetic organisms and are known for their intricate and diverse shapes. Diatoms are commonly found in freshwater and marine environments and play a significant role in the global carbon cycle.
Micrite is a fine-grained carbonate sedimentary rock composed of tiny carbonate particles. It forms through the precipitation and accumulation of carbonate minerals, such as calcite or aragonite, in marine environments. In the case of chalk, which is primarily composed of microscopic fragments of calcium carbonate from marine organisms, recrystallization can occur at the ocean bottom under specific conditions, leading to the formation of micrites.
Therefore, it's important to note that chert, marble, and quartzite are not the typical products of recrystallization of chalk at the ocean bottom.
For more details regarding diatoms, visit:
https://brainly.com/question/11446176
#SPJ4
You have 10.0 g each of na, c, pb, cu and ne. which contains the largest number of moles?
Answers
Carbon (C) contains the largest number of moles.
Number of Moles of a substance can be defined as the ratio of given mass of the substance to the Molecular Mass of the substance.
We can write,
Number of moles = Given Mass / Molecular Mass euation1
Given Mass of Na, C, Pb, Cu, Ne each = 10.0g
Molecular mass of Na = 22.9898 u
Molecular mass of C = 12u
Molecular mass of Pb = 207.2u
Molecular mass of Cu = 63.546 u
Molecular mass of Ne = 20.1797 u
After substituting the values of molecular mass and given mass of elements in equation1 , we will get
Number of moles of Na = (10/22.9898) = 0.4350
Number of moles of C = (10/12) = 0.8333
Number of moles of Pb = (10/207.2) = 0.0483
Number of moles of Cu = (10/63.546) = 0.1574
Number of moles of Ne = (10/20.1797) = 0.4955
So, we can clearly see that Carbon contains the largest number of moles.
To know more about Number of moles refer to the link:
https://brainly.com/question/13314627
#SPJ4
which of the following elements is the largest?
A. Boron
B. Nitrogen
C. Oxygen
D. Carbon
Answers
Answer:
A) Boron
Explanation:
I put oxygen and it was wrong lol. So, it said it was Boron
NEED HELP!! What types of waves are transmitted from the H-E-L-P device ?
Answers
Answer:
What do you mean need more details
Explanation:
Since a help device is a a communication gadget, we know that the help device will make use of radio waves.
What is a help device?A help device refers to a device that can be used for emergency communication. They are basically communication gadgets.
We must note that our communication gadgets such as television, radio, cell phone etc all make use of radio waves. Hence a help device would make use of radio waves.
Learn more about radio waves: https://brainly.com/question/3393755?
Fluorine, chlorine, and iodine are examples of
Answers
Answer:
Halogens
Explanation:
The halogens are a series of non-metal elements from group 17 of the periodic table (formerly VII). The halogens include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Answer:
halogens
Explanation:
just did the test on edg!
I
am having some difficulty with this lab work. im not really looking
for someone to do the work, but i need help with the formulas for
the variius parts. i also get that i will have to graph and use
7/7/12 Determination of Equilibrium Constant The purpose of this experiment is to determine the equilibrium constant, K., of the following equilibrium reaction. Duc 10 A CIL Fe³+ (aq) + SCN- (aq) = F
Answers
For the determination of equilibrium constant experiment, the purpose is to find the equilibrium constant (K) of the equilibrium reaction as follows: Fe³+ (aq) + SCN- (aq) = FeSCN²+ (aq)
The formulas that you need to know to complete this lab work are as follows:
Equilibrium constant,
Kc= [Products]^n/[Reactants]^m
where n and m are the stoichiometric coefficients of the products and reactants respectively; Concentration, c= n/V, where n is the amount of solute and V is the volume of solution; Molar extinction coefficient,
ε= absorbance/ (concentration * path length)
The first step for the lab is to prepare 0.200 M Fe(NO3)3 solution and 0.0020 M KSCN solution. After that, you will take 5.0 ml Fe(NO3)3 solution and add 5.0 ml of KSCN solution into it. You will take a blank solution with 10 ml distilled water. You will also take a reference solution of FeSCN²+ with known concentration. The solutions need to be mixed well to reach equilibrium.The next step is to measure the absorbance of the blank, reference, and sample solutions. The absorbance of the sample solution needs to be measured at 447 nm wavelength.Using the molar extinction coefficient and Beer’s law equation, you can find the concentration of FeSCN²+ in the sample solution. The concentration can then be used in the equilibrium constant equation to calculate the equilibrium constant, Kc.
You will repeat the experiment for several different Fe(NO3)3 and KSCN concentrations to obtain a set of data points. Then you can graph [FeSCN²+] vs. [Fe³+][SCN-] to obtain the equilibrium constant, Kc.
To know more about equilibrium visit:
https://brainly.com/question/30694482
#SPJ11
The equilibrium constant, K is an important property of a chemical system which helps in understanding the extent to which a reaction goes to completion. It is defined as the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium. The experiment to determine the equilibrium constant of a reaction requires a few formulas and a graph. The reaction being studied in this experiment is:
Fe³+ (aq) + SCN- (aq) ⇌ FeSCN²+ (aq)
To determine the equilibrium constant of this reaction, one must first prepare a set of solutions with different initial concentrations of Fe³+ and SCN-. The initial concentration of Fe³+ is fixed, and the initial concentration of SCN- is varied. Then, a small amount of Fe³+ is added to each solution, which reacts with SCN- to form FeSCN²+. The amount of FeSCN²+ formed is measured and recorded. This process is repeated for each solution, with a different initial concentration of SCN-. The concentration of FeSCN²+ at equilibrium for each solution is calculated using the following formula:
[FeSCN²+]eq = (Abs – (AεFeSCN²+))[FeSCN²+]eq = Abs - (AεFeSCN²+)
where Abs is the absorbance of the solution, A is the path length of the cuvette, and εFeSCN²+ is the molar absorptivity of FeSCN²+.
The equilibrium concentrations of Fe³+, SCN-, and FeSCN²+ can then be calculated using the initial concentrations and the amount of FeSCN²+ formed at equilibrium. Finally, the equilibrium constant of the reaction can be calculated using the equation:
K = [FeSCN²+]eq / ([Fe³+]eq [SCN-]eq)
To know more about equilibrium visit :
brainly.com/question/33298173
#SPJ11
Which of the following is NOT a feature that supports the particulate theory of matter?
A. There are empty spaces between the particles
B. The particles are in constant motion
C. There are no forces of attraction between the particles
D. Temperature has an effect on the speed of motion of the particles
Answers
There are no forces of attraction between the particles.
The kinetic energy of the molecule is greater than the attractive force between them, thus they are much farther apart and move freely from each other. In most cases, there are essentially no attractive forces between particles. This means that gas has nothing to hold a specific shape or volume.
Particles
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object to which can be ascribed several physical or chemical properties, such as volume, density, or mass. They vary greatly in size or quantity, from subatomic particles like the electron to microscopic particles like atoms and molecules, to microscopic particles like powders and other granular materials. Particles can also be used to create scientific models of even larger objects depending on their density, such as humans moving in a crowd or celestial bodies in motion.
The term particle is rather general in meaning and is refined as needed by various scientific fields. Anything that is composed of particles may be referred to as particulate. However, the noun particulate is most frequently used to refer to pollutants in the Earth's atmosphere, which are a suspension of unconnected particles, rather than a connected particle aggregation.
Learn more about Particles
https://brainly.com/question/18826360
#SPJ2
Assuming that the trends continue, which of the following compound will have the greatest solubility at 120 ℃? The graph below shows the solubility of a variety of compounds.
A. Ce2(SO4)3
B. K2Cr2O7
C. NaCl
D. Pb(NO3)2
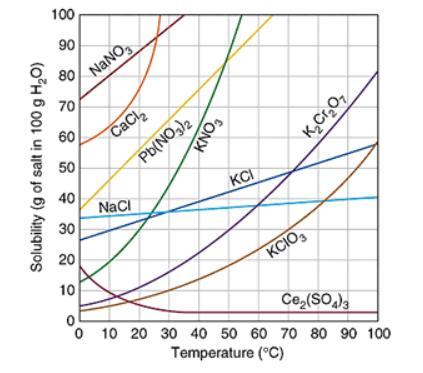
Answers
Answer:
its c
Explanation:
i took the test and got a 100
Assuming that the trends continue, sodium chloride compound will have the greatest solubility at 120 ℃.
What is solubility?Solubility is defined as the ability of a substance which is basically solute to form a solution with another substance. There is an extent to which a substance is soluble in a particular solvent. This is generally measured as the concentration of a solute present in a saturated solution.
The solubility mainly depends on the composition of solute and solvent ,its pH and presence of other dissolved substance. It is also dependent on temperature and pressure which is maintained.Concept of solubility is not valid for chemical reactions which are irreversible. The dependency of solubility on various factors is due to interactions between the particles, molecule or ions.
Learn more about solubility,here:
https://brainly.com/question/22185953
#SPJ3
This is on one of my homework assignments and I need help understanding this problem.

Answers
According to the electronic configuration, platinum is least reactive and is a noble metal.
What is electronic configuration?
Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.Elements undergo chemical reactions in order to achieve stability.
Learn more about electronic configuration,here:
https://brainly.com/question/29757010
#SPJ1
The most essential compound needed to sustain life as we know it is ________.
A) carbon dioxide
B) water
C) ozone
D) oxygen
E) carbohydrates
Answers
The most essential compound needed to sustain life as we know it is water. Therefore the correct option is option B.
Water is necessary for life for a number of reasons. It makes up a sizable portion of the human body and is essential for a variety of internal processes, such as controlling temperature, transferring nutrients and waste, and lubricating joints. Many other organisms depend on water for survival, and plants use it for photosynthesis.
Although it is likewise essential for life as we know it, oxygen is not regarded as a compound. Many species, including humans, require oxygen, an element, in order to breathe. Therefore the correct option is option B.
For such more question on compound:
https://brainly.com/question/28872356
#SPJ11
What volume of the stock solution (part a) would contain the number of moles present in the diluted solution (part b)? express your answer with the appropriate units.
Answers
The volume of the solution that we are looking for in the problem is 0.06 moles
What is a stock solution?A stock solution refers to a concentrated solution of a substance that is prepared with the intention of diluting it to obtain lower concentrations for various applications.
From part A;
Number of moles of luminol = 20g/177 g/mol
= 0.11 moles
Molarity = 0.11 moles * 1000/75 L
= 1.45 M
From part B;
Number of moles = Concentration * volume
= 0.03 M * 2L
= 0.06 moles
From part C;
Volume = Number of moles /Concentration
= 0.06 moles/1.45 M
= 0.04 L or 40 mL
Learn more about stock solution:https://brainly.com/question/33305064
#SPJ4

write the current equation at node 1 utilizing v1 and v2.
Answers
To determine the current equation at Node 1 using the voltages v1 and v2, we need to analyze the circuit connected to Node 1 and apply Kirchhoff's current law (KCL).
KCL states that the sum of currents entering a node must equal the sum of currents leaving the node. At Node 1, we can write the current equation as:
I1 = I2 + I3
Where I1 is the current at Node 1, I2 is the current entering Node 1 from a connected component, and I3 is the current leaving Node 1 to another connected component.
To express I1 in terms of v1 and v2, we can use Ohm's law and the relationship between voltage, current, and resistance. Let's assume that the connected components at Node 1 consist of resistors with values R2 and R3.
Using Ohm's law, we can write the following equations for I2 and I3:
I2 = v1/R2
I3 = v2/R3
Now, substituting these values into the KCL equation, we have:
I1 = v1/R2 + v2/R3
Thus, the current equation at Node 1, in terms of the voltages v1 and v2, is given by:
I1 = v1/R2 + v2/R3
This equation represents the relationship between the currents and voltages at Node 1, taking into account the resistances of the connected components. By knowing the values of R2 and R3, as well as the voltages v1 and v2, you can calculate the current I1 flowing through Node 1 in the circuit.
Learn more about Kirchhoff's current law :
https://brainly.com/question/15088107
#SPJ11
The Liquified Petroleum Gas (LPG) has the composition of 60% Propane (C 3
H 8
) and 40% Butane (C 4
H 10
) by volume: (a) Find the wet volumetric and gravimetric analysis of the products of combustion when the equivalence ratio (Φ)=1.0. (b) What is the stoichiometric air to fuel ratio for the LPG.
Answers
The balanced combustion reaction for propane can be represented as:
C₃H₈ + (5/2)O₂ → 3CO₂ + 4H₂O
And the balanced combustion reaction for butane can be represented as:
C₄H₁₀ + (6.5)O₂ → 4CO₂ + 5H₂O
Since LPG is composed of 60% propane and 40% butane by volume, we can calculate the wet volumetric and gravimetric analysis based on these proportions.
Wet volumetric analysis:
For the wet volumetric analysis, we consider the volume of the products of combustion relative to the volume of the LPG consumed.
Propane (C₃H₈):
The stoichiometric coefficient of propane in the combustion reaction is 3. Therefore, for every mole of propane burned, we will have 3 moles of CO₂ and 4 moles of H₂O formed.
Butane (C₄H₁₀):
The stoichiometric coefficient of butane in the combustion reaction is 4. Therefore, for every mole of butane burned, we will have 4 moles of CO₂ and 5 moles of H₂O formed.
Considering the initial composition of 60% propane and 40% butane by volume, we can calculate the volumetric composition of the products of combustion:
Volumetric composition of CO₂:
(0.6 * 3) + (0.4 * 4) = 3.6
Volumetric composition of H₂O:
(0.6 * 4) + (0.4 * 5) = 4.6
Therefore, the wet volumetric analysis of the products of combustion is 3.6 parts CO₂ to 4.6 parts H₂O.
Wet gravimetric analysis:
For the wet gravimetric analysis, we consider the mass of the products of combustion relative to the mass of the LPG consumed.
Using the molar masses of the compounds involved in the combustion reaction:
Molar mass of CO₂ = 44 g/mol
Molar mass of H₂O = 18 g/mol
Gravimetric composition of CO₂:
(0.6 * 3 * 44 g/mol) + (0.4 * 4 * 44 g/mol) = 158.4 g
Gravimetric composition of H₂O:
(0.6 * 4 * 18 g/mol) + (0.4 * 5 * 18 g/mol) = 74.4 g
Therefore, the wet gravimetric analysis of the products of combustion is 158.4 grams CO₂ to 74.4 grams H₂O.
(b) The stoichiometric air to fuel ratio for LPG can be determined based on the balanced combustion equations for propane and butane.
For propane (C₃H₈):
C₃H₈ + (5/2)O₂ → 3CO₂ + 4H₂O
The stoichiometric coefficient for propane is 1, which means we need 5/2 moles of O₂ for every mole of propane.
For butane (C₄H₁₀):
C₄H₁₀ + (6.5)O₂ → 4CO₂ + 5H₂O


