An atom that becomes charged due to the gain or loss of an electron is called an _____.
Answers
An atom that becomes charged due to the gain or loss of an electron is called an ion.
Ions are atoms or molecules that have an unequal number of protons and electrons, resulting in a net electrical charge. If an atom gains one or more electrons, it becomes negatively charged and is called an anion. If an atom loses one or more electrons, it becomes positively charged and is called a cation.
The charge of an ion depends on the number of electrons that are gained or lost. For example, if a sodium atom (Na) loses one electron, it becomes a sodium cation (Na+), with a net positive charge due to the unequal number of protons and electrons.
Ions play important roles in chemistry and biochemistry, as they can participate in a wide range of chemical reactions and biological processes. For example, metal ions such as calcium and magnesium are essential for many enzymatic reactions, while chloride and sodium ions are important for maintaining the balance of fluids and electrolytes in the body.
To know more about the Electron, here
https://brainly.com/question/18141448
#SPJ4
Related Questions
Na+ + Cl– Right arrow. NaCl
Which statement best describes the relationship between the substances in the equation?
Answers
Explanation:
I hope it helps
ya welcome

11. When a plant is growing, coming into view that is known as:
A.Photosynthesis
B. Emergence
C.Xylem
D. Phloem
Answers
Answer:
B
............. M
Explanation:
Emergence
How much solute can be dissolved in
200 mL of water at 30°C?
Answers
The maximum amount of NaCl that can dissolve in 200 mL of water at 30°C is 7.2 grams. It is important to note that this value may vary depending on the solubility of other solutes in the water, as well as factors such as pressure and the presence of other substances in the solution.
.Solubility is defined as the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure.
To determine the solubility of a solute, we can consult a solubility chart or database, which provides information on the solubility of different substances in water at different temperatures. For example, if we were to look up the solubility of sodium chloride (NaCl) in water at 30°C, we would find that it is approximately 36 grams per 100 mL of water.
Using this information, we can calculate the maximum amount of NaCl that can dissolve in 200 mL of water at 30°C. First, we convert 200 mL to 2/5 of 1000 mL, which is equivalent to 0.2 L. Then, we multiply the solubility of NaCl in water at 30°C (36 grams/100 mL) by the volume of water (0.2 L) to get the maximum amount of NaCl that can dissolve in 200 mL of water at 30°C:
36 g/100 mL x 0.2 L = 7.2 g
For more such questions on solubility
https://brainly.com/question/24057916
#SPJ11
presented with two tubes: one tube with a buffered solution + acid and one tube with water + acid, how will you know which tube has the buffer and which tube does not have the buffer?
Answers
One tube has a buffered solution + acid and the other tube has water + acid. To decide whether or not the solution is buffered, a simple pH test can be done. An acid-base indicator can be used to determine the pH of each solution.
A buffered solution is defined as a solution that can withstand minor changes in pH upon the addition of small amounts of an acid or base.
Consider the following steps:
To both tubes, add a small amount of acid-base indicator. Determine the pH of each solution by observing the color change of the acid-base indicator when it is added to it. The pH of the solution is determined by the color of the acid-base indicator after it has been added to it. Compare the pH of the two solutions. The solution with the lower pH is likely to have a buffer, whereas the solution with the higher pH is unlikely to have a buffer. This is due to the fact that the addition of an acid to a buffered solution would result in a lower pH, whereas the addition of an acid to an unbuffered solution would result in a higher pH. To find out which tube has the buffer and which does not, one has to compare the pH of each solution.Learn more about buffer: https://brainly.com/question/9458699
#SPJ11
Which of the following equations represents an acid-base neutralization reaction?
Group of answer choices
H2SO4 + Zn → ZnSO4 + H2
Ba(OH)2 + Na2SO4 → BaSO4 + 2NaOH
HCl + KOH → KCl + H2O
NaNO3 + KOH → KNO3 + NaOH
Answers
The equation HCl + KOH → KCl + H2O represents an acid-base neutralization reaction. Therefore, the equation that represents an acid-base neutralization reaction is HCl + KOH → KCl + H2O.
An acid-base neutralization reaction is defined as a type of chemical reaction in which an acid reacts with a base to produce salt and water. Here, the acid donates H+ ions and the base donates OH- ions. The net result is the neutralization of both acid and base.
HCl + NaOH → NaCl + H2O (hydrochloric acid and sodium hydroxide reacts to form sodium chloride and water).The above equation represents an acid-base neutralization reaction. Similarly, one of the equations provided in the question represents an acid-base neutralization reaction and it is: HCl + KOH → KCl + H2OThe remaining equations are:H2SO4 + Zn → ZnSO4 + H2 (single replacement reaction).Ba(OH)2 + Na2SO4 → BaSO4 + 2NaOH (double displacement reaction).NaNO3 + KOH → KNO3 + NaOH (double displacement reaction).
To know more about reaction visit:
https://brainly.com/question/30464598
#SPJ11
The directions on a box of egg dye tell you to add 3 tablespoons of vinegar to 1 cup of water before adding the
dye. Which of the following statements about your mixture?
a. The water is the solute and the vinegar is the solvent
b.
It is a heterogeneous mixture
C.
The water is the solvent and the mixture is the solute
d. It is a highly concentration mixture
Answers
A solution comprises solute and solvent that can be in the form of the same or different composition. The water is solvent and the mixture is solute. Thus, option c is correct.
What is a mixture?A mixture is a solution that may or may not have a definite and uniform composition. They even can have one or more phases. The mixture can be heterogeneous or homogeneous.
Here, the water is the solvent to which the solute, vinegar, and dye are added. The vinegar and dye both are liquid solutes that are added to the solvent to form a solution of definite composition.
Therefore, option c. mixture is the solute and water is the solvent.
Learn more about mixture here:
https://brainly.com/question/8647161
#SPJ1
I have ten moles of iron atoms in the reactant side of a reaction. Do I have enough information to determine how many moles of iron atoms I will have following the reaction? If so, how many moles of iron atoms will I have at the end of a reaction? If not, what else do I need to know?
Answers
Answer:
See below
Explanation:
If your reaction equation is balanced, you will know the number of Fe atoms following the reaction.....it will be the same on both sides of the equation.
What property of a metal does the image represent
Answers
Answer:
malleable
Explanation:
The image represent in malleable property of metal.
The image possibly represents the photoelectric effect of a metal, which is when it emits electrons after being exposed to electromagnetic radiation. Metals are also characterized by physical properties such as conductivity, malleability, metallic luster, and metallic bonding.
Explanation:Based on your question, the image possibly represents the photoelectric effect, a key property of metals. This phenomenon occurs when a metal surface exposed to electromagnetic waves of a certain frequency absorbs radiation and emits electrons. These emitted electrons are called photoelectrons. Metals can also exhibit free electron model behavior, where electrons freely roam within the metal structure.
Metals possess unique physical properties like conductivity, malleability, and metallic luster. Malleability refers to the metal's ability to deform without breaking, while conductivity refers to the metal's ability to transfer heat or electricity. A metallic luster gives metals their characteristic shiny appearance.
Finally, metals are also known for their metallic bonding—a unique force that holds together the atoms within a metallic solid. Metallic bonding gives rise to many useful and varied bulk properties of metals.
Learn more about Properties of Metals here:https://brainly.com/question/33514448
#SPJ2
Which quantity is held constant when working with Boyle's, Charles's, and Gay-Lussac's laws?
O volume
O moles
O pressure
O temperature
Answers
Answer:
moles
Explanation:
for these three principles, the volume, pressure and temperature of the particles will change whereas the moles will stay constant because the number of the particles does not change throughout the experiment.
Moles is held constant when working with Boyle's, Charles's, and Gay-Lussac's laws and the correct option is option 2.
What are Gas Laws?All gases generally show similar behaviour when the conditions are normal. But with a slight change in physical conditions like pressure, temperature or volume these show a deviation.
The gas laws are a group of laws that govern the behaviour of gases by providing relationships between the following:
The volume occupied by a gas.The pressure exerted by a gas on the walls of its container.The absolute temperature of the gas.The amount of gaseous substance (or) the number of moles of gas.Therefore, Moles is held constant when working with Boyle's, Charles's, and Gay-Lussac's laws and the correct option is option 2.
Learn more about Gas laws, here:
https://brainly.com/question/27009857
#SPJ5
N analyst prepared a sucrose solution by weighing 1kg of water and add 1.5kg sucrose.estimate the concentration of the resultant solution in (i) mass percent (ii) degree brix
Answers
The concentration of the solution in mass percent is 60%.
What is the mass percent?The concentration of a substance can be expressed in mass percent. This refers to the percentage of the solute that is contained in the solution.
Thus we can write;
Mass percent = Mass of solute/ Mass of solution * 100/1
Mass percent = 1.5kg/1.5kg + 1 Kg * 100/1
Mass percent = 60%
Hence, the concentration of the solution in mass percent is 60%.
Learn more about mass percent:https://brainly.com/question/5394922
#SPJ1
if during the interaction of 36.5 g of hydrochloric acid with magnesium 137 kg of joules energy are released, how much energy would be released if 96 g of magnesium acted
Answers
Explanation:
ANSWER
The molarity =
molecularweight
density×purity×10
=
36.5
1.18×36.5×10
=11.8molar
if an isotope lies above the band of stability on a plot of neutrons vs protons, it will decay via
Answers
If an isotope lies above the band of stability on a plot of neutrons vs protons, it will decay via beta-minus (β-) decay or electron capture.
The band of stability on a plot of neutrons vs protons represents the stable isotopes that exist naturally on Earth.
Isotopes that lie above this band have an excess of neutrons or protons and are typically unstable. These isotopes tend to decay in order to reach a more stable state.
Beta-minus decay occurs when a neutron in the nucleus converts into a proton and releases an electron and an antineutrino.
This process reduces the number of neutrons in the nucleus and increases the number of protons, moving the isotope closer to the band of stability.
Electron capture occurs when a nucleus captures an electron from an electron shell and combines it with a proton to form a neutron and a neutrino.
This process also reduces the number of protons in the nucleus and moves the isotope closer to the band of stability.
Other types of decay, such as alpha decay or beta-plus (β+) decay, may also occur for isotopes above the band of stability, depending on the specific properties of the isotope.
To know more about beta-minus decay, refer here:
https://brainly.com/question/31910313#
#SPJ11
what is chemical reaction
Answers
Answer:
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.
Explanation:
I hope it helps! Please mark my answer as a bräinliest.
Thank You!
Explain why the mass of the powder is greater than the mass of the magnesium metal.
Answers
Answer:
because when it burns reacts with oxygen forming mg oxide
1. What is the density of a strip of magnesium that occupies a volume of 8.49 mL and weighs
33.7 g.
I
Answers
Answer:
The answer is 3.97 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass of metal = 33.7 g
volume = 8.49 mL
So we have
\(density = \frac{33.7}{8.49} \\ = 3.969375736...\)
We have the final answer as
3.97 g/mLHope this helps you
will give brainlist!!!
21. Which answer best describes the coast?
A. thick deposits of sediments carried off of the shelf
B. the surf area along coastlines
C. 75 mile shallow flat area just off coastlines
D. area of land that drops toward deep ocean basins
Answers
Answer:
C
Explanation:
75 mile shallow flat area just off coastlines
a light bulb consumes energy at a rate of 80.0 joules per second. how long in seconds will it take for the light bulb to consume 2.30x10
Answers
The light bulb will take approximately 2.9 x 10² seconds to consume 2.30 x 10² joules of energy.
To calculate the time it takes for the light bulb to consume a given amount of energy, we can use the formula:
Time (in seconds) = Energy Consumed / Energy Consumption Rate
Given that the energy consumption rate of the light bulb is 80.0 joules per second, and we want to find the time it takes for the light bulb to consume 2.30 x 10² joules of energy, we can substitute these values into the formula:
Time = (2.30 x 10² joules) / (80.0 joules per second)
Time = 2.875 x 10² seconds
Time ≈ 2.9 x 10² seconds
learn more about Energy here:
https://brainly.com/question/31434118
#SPJ11
If you start with 0.030 M of I2 at this temperature, how much will remain after 5.12 s assuming that the iodine atoms do not recombine to form I2 ?
Answers
If the iodine atoms do not recombine to form I2, then the reaction that is taking place is I2 → 2I. This reaction is first order, which means that the rate of the reaction depends on the concentration of I2.
The rate law for this reaction is:
Rate = k[I2]
where k is the rate constant for the reaction.
To solve for the amount of I2 remaining after 5.12 s, we need to use the integrated rate law:
ln([I2]t/[I2]0) = -kt
where [I2]t is the concentration of I2 at time t, [I2]0 is the initial concentration of I2, k is the rate constant, and t is the time.
Rearranging this equation gives:
[I2]t = [I2]0 * e^(-kt)
We can find k by using the half-life of the reaction, which is 1.76 s at this temperature.
t1/2 = ln2/k
k = ln2/t1/2
k = ln2/1.76 s
k = 0.393 s^-1
Now we can plug in the values and solve for [I2]t:
[I2]t = 0.030 M * e^(-0.393 s^-1 * 5.12 s)
[I2]t = 0.018 M
Therefore, after 5.12 s, 0.018 M of I2 will remain assuming that the iodine atoms do not recombine to form I2.
To Learn more about iodine atoms. Click this!
brainly.in/question/43901490
#SPJ11
What is morning wood?
Answers
Answer:
nocturnal penile tumescene
Explanation:
is a spontaneous erection of penis during sleep or when waking up.
Please help
How many moles of O2 are required to generate 12 moles SO2 gas? 2CuFeS2 + 502 → 2Cu + 2FeO + 4SO2 [ ? ] mol O₂ O2
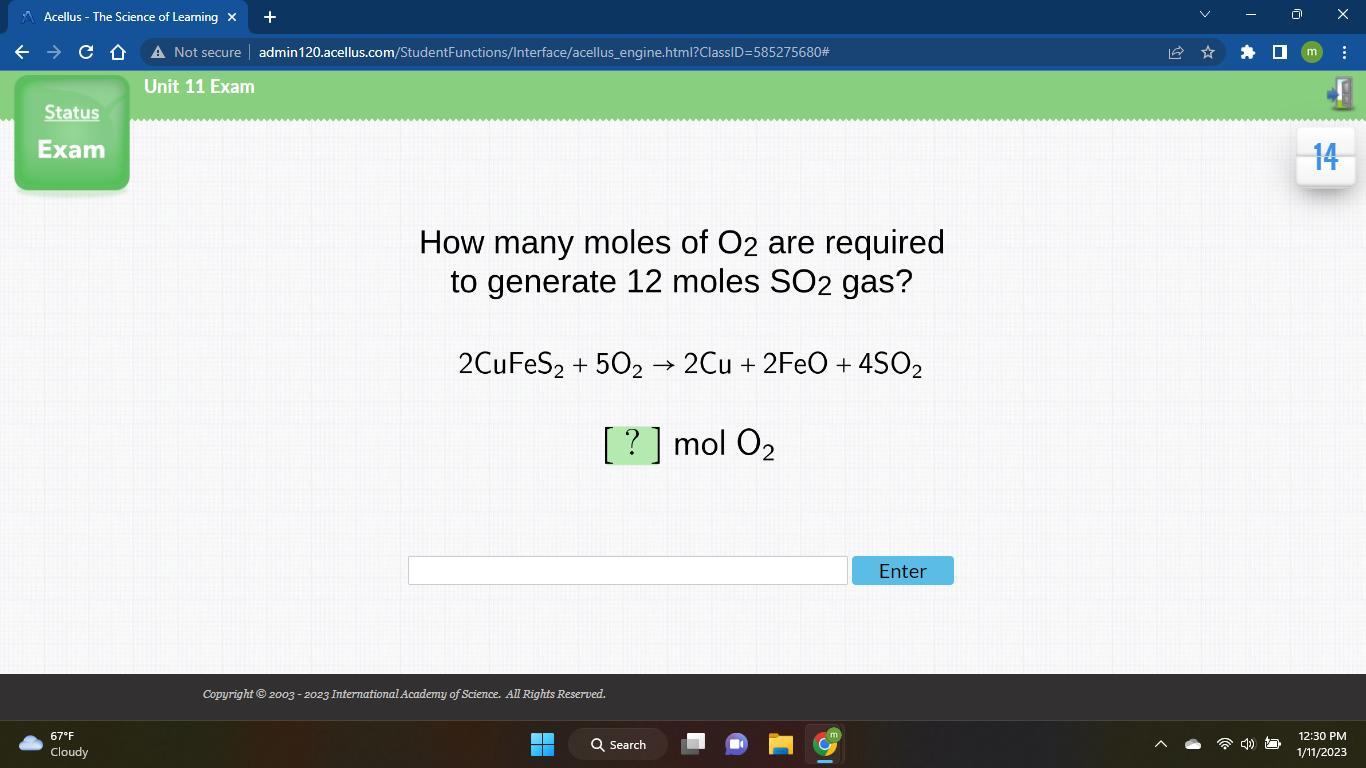
Answers
Answer:30 moles of oxygen are required if 12 moles of are consumed.
Explanation:The given balanced equation is:
From this balanced equation, there is 2:5 mol ratio between and .
We are asked to calculate the moles of required to react with 12 moles of .
It's a mol to mol conversion and the set up for this would be as:
=
So, 30 moles of oxygen are required to react with 12 moles of .
what semisolid dosage form is of stiff consistency and contains a large proportion of solids finely disepred in a fatty vehicle
Answers
The semisolid dosage form that is of stiff consistency and contains a large proportion of solids finely dispersed in a fatty vehicle is a cream.
What are the semisolid dosage forms?Semisolid dosage forms are a class of preparations that consist of a small amount of aqueous and/or oleaginous liquid and a larger quantity of solid materials. The solid phase of these preparations contains a variety of substances, including petrolatum, paraffin, stearic acid, cetyl alcohol, and others.
These dosage forms include ointments, creams, gels, pastes, and suppositories, all of which are designed to be applied to the skin or mucous membranes to achieve a local or systemic effect.
The cream is a semisolid dosage form that is of stiff consistency and contains a large proportion of solids finely dispersed in a fatty vehicle. Creams are often used for topical application to the skin, and they may be used to treat a variety of skin conditions, including dry skin, psoriasis, and eczema.
Creams have a lower viscosity than ointments and are therefore easier to apply to the skin. They also contain more water than ointments, which allows them to be absorbed into the skin more quickly.
learn more about semisolid dosage here
https://brainly.com/question/26051956
#SPJ11
A ball rolled down from the top of the slope. What kind of energy
conversion occurs from the top to bottom of the inclined plane?
a Kinetic to gravitational potential
b Elastic potential to kinetic
C Gravitational potential to kinetic
d Internal energy to kinetic
e Electric to kinetic
Answers
Answer:
C
Explanation:
The ball is undergoing a gravitational potential to kinetic energy transformation. When the ball is at the top of the slope, it has the potential to be moved down by gravity, hence the name. When it's rolling down the slope, it turns to kinetic energy, which is when something moves.
Suppose the isotopic ratio of the two boron isotopes 10B (10.013 amu) and 11B (11.009 amu) in a sample has been altered from the ratio found in nature and now contains 33.36% 10B in the sample. Determine the atomic weight of this sample of this new boron element.
Answers
Answer:
The correct answer is 10.676 amu.
Explanation:
Based on the given information, the concentration of 10B left in the sample is 33.36%. Therefore, the percentage of 11B present will be,
11B = 100% - 33.36% = 66.64%
Now the atomic weight of the new boron element can be determined by adding the atomic masses of both the isotopes multiplied by its percentage.
Therefore,
= (10.013 amu * 33.36%) + (11.009 amu * 66.64%) / 100
= 10.676 amu
3. Which of the following provides the best explanation for why the water drop does not slide off the inclined plane?
90 80 70
50
40
30
10
O A The polar water molecules are absorbed by the underlying surface.
OB The polar water molecules cause the surface to become temporarily charged, causing adhesion
OC The polar water molecules exert strong cohesive forces on one another
OD. The polar water molecules are repelled by the nonpolar surface
Answers
Answer:
The polar water molecules exert strong cohesive forces on one another
Explanation:
The forces of cohesion refer to the strong attractive forces that molecules of a substance exert on each other.
This strong attractive force keeps the molecules of the water together and causes the water molecules to be pulled inside towards each other. We refer to this phenomenon as surface tension.
Hence, due to surface tension, water does not run off an inclined plane.
Explain how the cohesive and adhesive properties of water are useful in maintaining various life processes.
Answers
Answer:
Water molecules' adhesion aids plants in moisture absorption at their roots. Water's initial boiling point is attributed to cohesion, which helps animals regulate their body temperature.
Explanation:
2. Complete these sentences about single atoms. a. Atomic number is the number of ...... b. Mass number is the number of ........ c. Another name for mass number is
Answers
Answer:
The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (np) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons.
For an ordinary atom, the sum of the atomic number Z and the neutron number N gives the atom's atomic mass number A. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity called the "relative isotopic mass"), is within 1% of the whole number A.
How much calcium is in 8 g of calcium? Answer in units of MOL
Answers
Answer:
0.199610759019912
Explanation:
Sorry, couldn't make it a whole number
Which statement describes a chemical property of
silicon?
(1) Silicon has a blue-gray color.
(2) Silicon melts at 1414 C.
(3) Silicon reacts with fluorine.
(4) Silicon is a brittle solid at 20."C.
Answers
everything describes physical traits ect. and 3 explains reactions with a different substance so 3
when carbon is heated in a limited supply of oxygen, a gas is obtained.
1 .what is the name of this gas
Answers
Answer:
carbon monoxide
I think its correct but I am not sure
Answer: when carbon is heated in air carbon dioxide is formed, so is incomplete combustion which results in carbon monoxide.
Explanation: but when carbon dioxide reacts with more oxygen carbon monoxide is formed i guess.
I AM A BIT SURE BUT HOPE THIS HELPSSSS!!!!
Molar mass if calcium nitrate
Answers
Answer:
About 164 grams
Explanation:
The molecular formula of calcium nitrate is \(Ca(NO_3)_2\). The molar mass of calcium is about 40, while the molar mass of nitrogen is about 14 and oxygen is about 16. Therefore, the molar mass of it is:
\(40+2(14+3(16))=40+2(14+48)=40+124=164\)
Hope this helps!