An erlenmeyer flask containing 20. 0 ml of sulfuric acid of an unknown concentration was titrated with exactly 15. 0 ml of 0. 25 m naoh solution to the equivalence point. What was the concentration of sulfuric acid in the flask?.
Answers
The concentration of sulfuric acid in the flask is 0.25 M
The volume of NaOH solution required to reach the equivalence point is 15.0 mL. The objective is to determine the concentration of sulfuric acid in the flask.
The balanced chemical equation for the reaction is:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Let's use M1V1 = M2V2 equation to find the concentration of the sulfuric acid.
M1 is the concentration of the Sodium Hydroxide solution,
V1 is the volume of the NaOH solution used for titration.
V2 is the volume of the Sulfuric acid solution, and
M2 is the unknown concentration of the sulfuric acid solution.
So, the equation becomes:0.25 M × 15.0 mL = M2 × 20.0 mL
The volume of the NaOH solution required to reach the equivalence point is 15.0 mL.
Therefore, the volume of sulfuric acid in the Erlenmeyer flask is also 15.0 mL.
The equation now becomes:0.25 M × 15.0 mL = M2 × 15.0 mL 0.25 M = M2
Thus, the concentration of the sulfuric acid solution is 0.25 M.
To know more about concentration, click here
https://brainly.com/question/3045247
#SPJ11
Related Questions
The titration should be stopped when the solution in the flask changes color and the new color remains even when the solution is gently shaken. True False
Answers
The above given statement is true.
What is solution?A homogeneous mixture comprised of two or more components is referred to as a solution. Because they are consistently scattered at the molecular or ionic level, the components in a solution are evenly mixed and have a constant composition.
Until the reaction between the two solutions is complete, a solution of known concentration is gradually added to a solution of unknown concentration in a titration. The endpoint, or point at which the reaction is finished, is often identified by a color change that takes place after an indicator is added to the solution being titrated.
The reaction has achieved its endpoint when the solution in the flask changes color, and the new color persists even after the solution is gently shaken. At this time, the titration should be halted. By doing this, it is possible to precisely determine the concentration of the unknown solution and the volume of titrant added to the solution.
To know more about solution, visit:
https://brainly.com/question/30665317
#SPJ1
At stp,250cm3 of gas had a mass of 0.36g.what result does this give for the molar mass of the gas
Answers
Answer:
32,256g/mol
Explanation:
n= V/Vm
n = 0.25/22.4
n = 0.01
0.01 = 0.36/M
M = 0.36/0.01
why is enol formation in dimedone more favorable than in cyclohexanone?
Answers
The formation of enol is more favorable in dimedone compared to cyclohexanone due to the presence of keto-enol tautomerism and the stabilization of the enol form.
In dimedone, the presence of two electron-withdrawing carbonyl groups (C=O) on adjacent carbon atoms allows for the formation of an enol tautomer. The enol form is stabilized through intramolecular hydrogen bonding between the hydrogen atom of the enol group and the oxygen atom of the neighboring carbonyl group. This hydrogen bonding imparts additional stability to the enol form, making its formation more favorable. On the other hand, cyclohexanone lacks the electron-withdrawing carbonyl group in close proximity to facilitate enol formation. The absence of adjacent carbonyl groups hinders the formation of the enol tautomer and reduces the stability of the enol form. As a result, the formation of enol is less favorable in cyclohexanone compared to dimedone.
To learn more about Keto-enol tautomerism click here ; brainly.com/question/15411301
#SPJ11
Calculate the standard reaction enthalpy for the reaction below:
3Fe2O3(s) → 2Fe3O4(s) + ½O2(g)
Answers
The standard reaction enthalpy for the given reaction is +235.8 kJ/mol.
What is the standard reaction enthalpy of reaction?The standard reaction enthalpy (ΔH°) for the given reaction is determined as follows:
Equation of reaction: 3 Fe₂O₃ (s) → 2 Fe₃O₄ (s) + ½ O₂ (g)
The standard enthalpy of formation values for Fe₂O₃ (s), Fe₃O₄(s), and O₂(g) is used to calculate the standard reaction enthalpy.
ΔH° = [2 × ΔH°f(Fe₂O₃)] + [½ × ΔH°f(O₂)] - [3 × ΔH°f(Fe₃O₄)]
where;
ΔH°f(Fe₂O₃) = -824.2 kJ/mol
ΔH°f(Fe₃O₄) = -1118.4 kJ/mol
ΔH°f(O₂) = 0 kJ/mol
ΔH° = [2 × (-1118.4 kJ/mol)] + [½ × 0 kJ/mol] - [3 × (-824.2 kJ/mol)]
ΔH° = -2236.8 kJ/mol + 0 kJ/mol + 2472.6 kJ/mol
ΔH° = 235.8 kJ/mol
Learn more about standard reaction enthalpy at: https://brainly.com/question/15174388
#SPJ1
When the fish water had pH of 8.0, the hydronium ion concentration is 1.0x 10^-8 mole per liter. What is Hydornium ion con ent ration when water had pH of 7.0
Answers
The hydronium ion concentration when pH = 7 is : 8.75 * 10⁻⁹ mole/liter
Determine hydronium ion concentration when pH = 7
Given that :
pH = 8.0
Hydronium ion concentration = 1 * 10⁻⁸ mole/liter
Resolving the question
8.0 = 1 * 10⁻⁸ mole/liter
7.0 = x
therefore :
x = 7 ( 1 * 10⁻⁸ ) / 8.0
= 8.75 * 10⁻⁹ mole/liter
Hence we can conclude that The hydronium ion concentration when pH = 7 is : 8.75 * 10⁻⁹ mole/liter
Learn more about hydronium ion : https://brainly.com/question/27038951
#SPJ1
based on figure 1, which of the following statements best predicts the effect that a change from a moderately acidic environment (phph near 6) to a basic environment will have on peroxidase activity?
Answers
Peroxidase activity increases and then decreases due to a change from a moderately acidic environment (pH near 6) to a basic environment.
What is peroxidase activity?Peroxidase is an enzyme found in a wide variety of organisms, from plants to humans to bacteria. Its function is to decompose hydrogen peroxide (H₂O₂). Hydrogen peroxide is one of the toxins produced as a by-product of using oxygen to breathe. (The fact that hydrogen peroxide is toxic makes it useful in first aid kits.
Peroxidase activity is strongly influenced by pH factors. Peroxidase works best at pH 7, but increasing or decreasing pH adversely affects its activity. Therefore, when moving from a weakly acidic environment close to pH 6 to a basic one, the peroxidase activity increases as the neutral environment approaches and decreases as the basic environment approaches, so that the peroxidase activity varies with the pH of the solution.
To know more about peroxidase, visit:
https://brainly.com/question/14870911
#SPJ1
The complete question is as follows:
Researchers investigated the influence of environmental pH on the activity of peroxidase, an enzyme that catalyzes the conversion of hydrogen peroxide to water and oxygen gas. In an experiment, the researchers added a hydrogen peroxide solution containing guaiacol to several identical test tubes and adjusted the solution in each test tube to a different pH . The researchers included the guaiacol because it caused the solutions to change color as the reactions proceeded, which the researchers relied on for measuring reaction rates. Finally, the researchers added the same amount of peroxidase to each test tube and measured the rate of each reaction at 23°C . The results of the experiment are represented in Figure 1.
Based on Figure 1, which of the following statements best predicts the effect that a change from a moderately acidic environment ( pH near 6) to a basic environment will have on peroxidase activity?
answer choices
Peroxidase activity will decrease.
Peroxidase activity will increase.
Peroxidase activity will stay the same.
Peroxidase activity will increase at first and then decrease.
explain why an rh-negative person doesn't have a transfusion reaction on the 1st exposure to rh-positive blood but does have a reaction on the 2nd exposure.
Answers
The first occasion a Rh-negative patient receives Rh-positive blood, a transfusion reaction does not happen also because Rh-negative patient has not had time to develop antibodies against by the Rh-positive blood.
Transfusion response definition:Some patients can have negative reactions towards the plasma they obtain after a donation, even when the right blood type is used. Some of the symptoms under these circumstances include hives and itching. Like other allergic reactions, this can be managed with medications. However, a professional should be sought if the reaction gets worse.
Which transfusion response is the worst?An acute immune hemolytic event, a very hazardous but unusual occurrence, can occur when the immune system rejects the directly injected red blood cells. The attack causes the discharge of a substance that damages the kidneys.
To know more about transfusion reaction visit:
https://brainly.com/question/13253562
#SPJ4
What is the specific heat of an unknown metal of 2.93 kcal of energy are required to raise the temperature of 183.4g sample of the metal by 126.3 Celcius
Answers
Answer:
The specific heat of the unknown metal is 1.26*10⁻⁴ \(\frac{kcal}{g* C}\)
Explanation:
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Sensible heat occurs when heat added or removed from a substance causes a temperature change in it and is calculated by:
Q = c * m * ΔT
Where Q is the heat exchanged by a body of mass m, constituted by a substance of specific heat c and where ΔT (Tfinal - Tinitial) is the variation in temperature
In this case:
Q= 2.93 kcalc= ?m= 183.4 gΔT= 126.3 °CReplacing:
2.93 kcal= c* 183.4 g*126.3 °C
Solving:
\(c=\frac{2.93 kcal}{183.4 g* 126.3 C}\)
c= 1.26*10⁻⁴ \(\frac{kcal}{g* C}\)
The specific heat of the unknown metal is 1.26*10⁻⁴ \(\frac{kcal}{g* C}\)
Which of the following is true?
- [ ] A mole of propane has more mass than a gram of propane.
- [ ] A mole of propane has 6.03 x 10^23 grams.
- [ ] A gram would have a lot more molecules of propane than a mole.
- [ ] There’s no difference; they’re the same.
Answers
Answer:
The answer should be D
Explanation:
because turning 1 mole of propane to grams means its still one mole of propane just in a different unit.
In the redox reaction AgNO3 + Na → NaNO3 + Ag, which element has been
oxidized?
Answers
Answer:
sodium I think .............
Which of the following correctly describes a compound? (4 points)
The atoms are chemically bonded together, and they retain their individual physical and chemical properties.
The atoms are not chemically bonded, and there is no set ratio for how the atoms can combine together.
The atoms can only combine in fixed ratios, and they can only be separated by a chemical change.
The atoms do not retain their individual chemical properties, and they can be separated by physical means.
Answers
Explanation:
The atoms are chemically bonded together, and they retain their individual physical and chemical properties.
Answer:
The atoms can only combine in fixed ratios, and they can only be separated by a chemical change.
Explanation:
just took the test the other guys thing was wrong
One pound of weight gain comes from eating 3500 calories more than what you burn. A can of soda contains 150 calories. If you drink 2 sodas every day, how many pounds will gain after a year? (Assume the soda calories are your only “extra” calories.)
Answers
Answer:
31.3 pounds
Explanation:
300x365=109,500 109,500/3500=31.3 or (31 if you round)
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
Individual solute particles are broken apart from the solid by these particles. * a Solution b Insoluble c Solvent d Compounds
Answers
Answer:
Individual solute particles are broken apart from the solid by the;
c. Solvent
Explanation:
A solution is the homogeneous mixture that is made up of two or more substances formed by dissolving a substance which can be a solid, liquid or gas in another substance known as the solvent which normally the larger part of the fraction of the solution than the solute and can also be a solid, liquid or a gas
In a solution the solvent particles serves to brake of and disperser parts of a solid solute to form a more or less homogeneous mixture
Therefore, the solute particles are broken by the solvent particles in a solution
A solution has [OH-]=3.5*10^-6. Based on that, what must be true about this solution?
![A solution has [OH-]=3.5*10^-6. Based on that, what must be true about this solution?](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/YW5TyfgGVLOydEVPBNUxrU45MizSGS3C.jpeg)
Answers
Answer:
Option B. It is a basic solution.
Explanation:
To know which option is correct, let us calculate the pH of the solution.
First, we shall determine the pOH of the solution. This is illustrated below:
Concentration of Hydroxide ion, [OH¯] = 3.5×10¯⁶
pOH =.?
pOH = –Log [OH¯]
pOH = –Log 3.5×10¯⁶
pOH = 5.5
Finally, we shall determine the pH. This can be obtained as follow:
pOH = 5.5
pH =?
pH + pOH = 14
pH + 5.5 = 14
Collect like terms
pH = 14 – 5.5
pH = 8.5
The pH scale reads as follow:
0 to 6 => Acidic
7 => Neutral
8 to 14 => Basic
Comparing the pH of the solution (i.e 8.5) with the pH scale, we can conclude that the solution is basic because the pH of solution lies between 8 and 14.
identify the most likely cause of earthquakes that occur in the area shown on the map

Answers
The most likely cause of earthquakes that occur in the area shown on the map is due to fault lines in the earth's crust.
What are earthquakes?Earthquakes are natural phenomena characterized by the shaking or trembling of the Earth's surface.
They occur due to the sudden release of energy in the Earth's crust along fault lines, which creates seismic waves that propagate through the Earth.
The Earth's crust is composed of several large tectonic plates that float on the semi-fluid layer of the Earth's mantle.
Learn more about earthquakes at: https://brainly.com/question/248561
#SPJ1
What elements and how many of each are in silicon dioxide, SiO2 (sand)?
Answers
Answer:
Silica (quartz): Silica, SiO2, is a chemical compound that is composed of one silicon atom and two oxygen atoms. It appears naturally in several crystalline forms, one of which is quartz.
Explanation:
why are protons (h+) pumped across the inner mitochondrial membrane?
Answers
Protons (H⁺) are pumped across the inner mitochondrial membrane as part of the process called electron transport chain, which is an essential step in cellular respiration.
The electron transport chain is responsible for generating adenosine triphosphate (ATP), the main energy currency of cells.
During cellular respiration, electrons are transferred from high-energy molecules (such as glucose) through a series of electron carriers embedded in the inner mitochondrial membrane. As electrons pass through the electron transport chain, energy is released and used to pump protons (H⁺) from the mitochondrial matrix to the intermembrane space.
There are some several reasons;
Establishing an Electrochemical Gradient; The pumping of protons across the inner mitochondrial membrane creates an imbalance of protons, resulting in a higher concentration of protons in the intermembrane space compared to the matrix.
Generation of ATP; The electrochemical gradient created by the proton pumping is utilized by ATP synthase, an enzyme complex embedded in the inner mitochondrial membrane.
Coupling Electron Transport with Proton Pumping; The pumping of protons across the inner mitochondrial membrane is coupled with the flow of electrons through the electron transport chain.
To know more about mitochondrial membrane here
https://brainly.com/question/31808640
#SPJ4
Which reaction will most likely take place based on the activity series? li > k > ba > ca > na > mn > zn > cr > fe > cd > ni > h > sb > cu > ag > pd > hg > pt pt fecl3 right arrow. mn cao right arrow. li znco3 right arrow. cu 2kno3
Answers
This outcome is conceivable. Since Cr has a lower reactivity than K, it cannot remove K from its complex.
How are reactants categorised?Condensation reactions (and their opposite, cleavage reactions), exchange reactions, acid-base interactions, and oxidation-reduction processes are the five categories into which the majority of chemical reactions can be divided.
The five main categories into which the bulk of chemical reactions can be grouped are acid-base reactions, exchange reactions, condensation reactions (and its opposite, cleavage reactions), and oxidation-reduction reactions.
The classification strategy is purely for convenience; a reaction may be classified in a number of ways depending on which of its characteristics is most important. Condensation processes are covered in this section.
For more information about reactants, visit
brainly.com/question/14225536
#SPJ4
Answer:
C
Explanation:
Li + ZnCO3 -->
question 13 help i’m timed thx u r loved

Answers
Answer:
178.3 L
Explanation:
178,300/1000
Why is making proteins important
Answers
which type of radioactivity has a negative charge?
A) alpha, α.
B) beta, β. C) gamma, γ. D) delta, δ
Answers
Answer:
Beta β
Explanation:
Beta particles are negatively charged electrons emitted by the nucleus on decay
Which statement is true of the energy levels of electrons in shells.
Answers
The correct statements about the energy levels of electrons in shells are the following:
Each electron shell has a different energy level.The electron shells closer to the nucleus are of lower energy than those further away.What are the energy levels of electrons?They are the energy charge that an electron possesses, where in each period (in each shell) only a certain number of electrons fit.
Characteristics of energy levels of electronsEach orbit corresponds to an "energy level" and can only contain a strictly defined number of electrons.Within each energy level, electrons could be grouped into "sublevels," and each sublevel could only contain a given number of electrons.The electrons are placed in the different levels and sublevels in order of increasing energy until they are completed.Therefore, we can conclude that the electrons of an atom have different energy levels, so they are classified by the energy level in which each of them is located.
Learn more about the energy levels of electrons here: https://brainly.com/question/19362949
Aluminum has a density of 2.70 g/mL. A sample of aluminum weighs 67.5 g. If this sample is put into a graduated cylinder that has 35 mL, what volume will the water rise to?
Answers
Answer:
60 ml
Explanation:
Now, we know that density = mass/volume
Mass of aluminium = 67.5 g
volume of aluminium =?
density of aluminium = 2.70 g/mL
volume of aluminium = mass/density = 67.5/2.70 = 25 ml
Since volume of water in the cylinder = 35 ml
The water rises to ; 35 ml + 25 ml = 60 ml
Someone help me out with this brain pop ⚠️
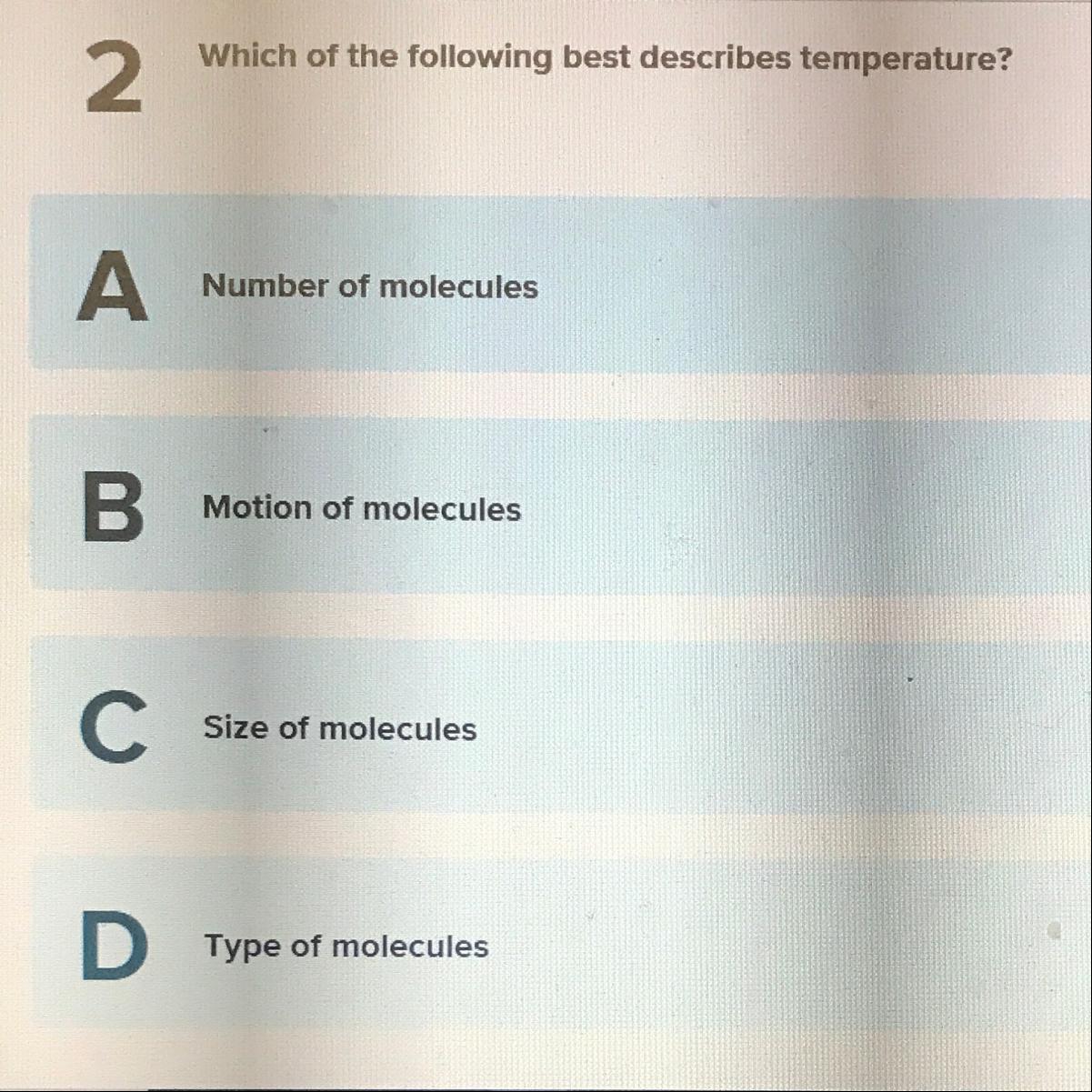
Answers
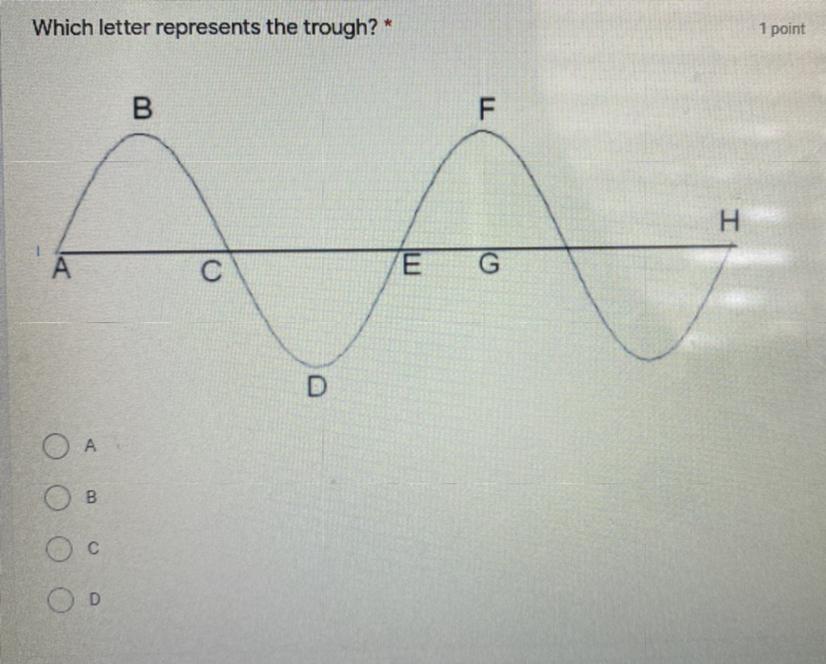
What letter represents the trough? :) PLEASE FAST!
1) A
2) B
3) C
4) D
Answers
A gaseous compound is subjected to increased pressure. What is happening to the temperature at the same time?-increased temperature, increased volume-increased temperature, decreased volume-decreased temperature, increased volume-decreased temperature, decreased volume
Answers
A gaseous compound is subjected to increased pressure. What is happening to the temperature at the same time?
We assume the gas is in a container.
If we increase the pressure, is a direct relationship with temperature, so the temperature increases, but if the volume is not constant, it will decrease.
Answer: increased temperature, decreased volume.
Using collum e create a blanace sheet for the year prior on Part C
Answers
To create a balance sheet for the year prior on Part C using column E, list the assets and liabilities in column E, calculate the total assets and total liabilities, and then subtract the total liabilities from the total assets to find the owner's equity. To create a balance sheet for the year prior on Part C using column E, follow these steps:
1. Start by listing all the assets in column E. Assets are items of value owned by the company. Examples of assets include cash, accounts receivable, inventory, and property. Enter the values of each asset in column E.
2. Next, list all the liabilities in column E. Liabilities are the debts and obligations of the company. Examples of liabilities include accounts payable, loans, and taxes payable. Enter the values of each liability in column E.
3. Calculate the total assets by adding up the values in column E for all the assets.
4. Calculate the total liabilities by adding up the values in column E for all the liabilities.
5. Subtract the total liabilities from the total assets to find the owner's equity. Owner's equity represents the owner's investment in the company and is calculated as the difference between assets and liabilities.
6. Finally, list the owner's equity in column E.
To know more about balance sheet visit :
https://brainly.com/question/33094018
#SPJ11
To which family on the Periodic Table does this Bohr Model belong?

Answers
Answer: Hydrogen
Explanation: The Bohr Atom is a very simplified model of the electron positions of each element of the Periodic Table. Each Row of the periodic table is represented by an orbit. Hydrogen and Helium are in the first energy level (row) of the periodic table and their Bohr Models would have one orbit.
Hope this helps! :)
evidence of a chemical change includes A) change in size B) change in color C) change in shape D) change in weight
Answers
Answer:
B) change in color
Explanation:
The evidence of a chemical change includes a change in color. Thus, the correct option for this question is B.
What is a Chemical change?A chemical change may be defined as a type of change that significantly involves the transformation of one chemical substance into one or more different substances. It type of change typically produces a new substance that is not earlier present during the reaction.
The attributes like change in size, change in shape, and change in weight demonstrate evidence of physical change. While the change in color completely transforms its appearance as compared to the previous.
It includes examples like burning of coal, rusting of iron, boiling of eggs, digestion of food, etc.
Therefore, the change in color is evidence of a chemical change. Thus, the correct option for this question is B.
To learn more about Chemical changes, refer to the link:
https://brainly.com/question/1334812
#SPJ6