Based on the behaviors of acids and bases, identify which procedures you would have used to examine an unknown acid that would also be applicable to examining an unknown base.
Check ALL THAT APPLY.
**determining the molar mass
**measuring the pH
**diluting the sample solutions to characterize as a weak or strong base
**isolating a sodium salt of the weak base
**determining the equilibrium constant
Answers
To examine an unknown acid and an unknown base, the following procedures can be used:
1. Measuring the pH: This procedure can be used to determine whether the substance is an acid or a base. Acids have a pH less than 7, while bases have a pH greater than 7.
2. Diluting the sample solutions to characterize as a weak or strong base: This procedure can be used to determine whether the substance is a weak or strong base. Weak bases do not completely dissociate in water, while strong bases do.
3. Determining the equilibrium constant: This procedure can be used to determine the strength of an acid or a base. The equilibrium constant is a measure of how much of the acid or base dissociates in water.
Therefore, the procedures that are applicable to examining both an unknown acid and an unknown base are measuring the pH, diluting the sample solutions to characterize as a weak or strong base, and determining the equilibrium constant.
Related Questions
The formation of the silver(I) ammine complex ion is a reversible reaction that is allowed to reach equilibrium. For each subsequent change to the system, indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration and leaving t blank means there is no change in the concentration Ag+(aq) + 2NH3 (aq)-----------------> Ag(NH3 (aq) <----------------- increasing the concentration of Ag+ decreasing the concentration of NH increasing the concentration of Ag(NH)
Answers
Explanation:
Ag+(aq) + 2NH3 (aq) ⇄ Ag(NH3)2 (aq)
When it comes to question of this sort, the LeChatelier principle should come to mind. The LeChatelier principle states that changes in the temperature, pressure, volume, or concentration of a system will result in predictable and opposing changes in the system in order to achieve a new equilibrium state.
increasing the concentration of Ag+
This would lead to an increase in concentration of reactants, the system would annul or oppose this change by moving towrds the right, favouring the forward reaction.
decreasing the concentration of NH3
This would lead to a decrease in concentration of reactants, the system would annul this change by moving to the left, favourin the backward reaction.
increasing the concentration of Ag(NH3)2
This would lead to an increase in concnetration of products, the system would annul or oppose this change by moving towards the left, favouring the backward reaction.
A 2.0 mL sample of carbon tetrachloride (CCI4 ) masses at 3.16 g. What is the density of this substance in g/mL?
A. 1.5 g/mL
B. 1.58 g/mL
C. 1.6 g/ml
D. 0.63 g/ml
Answers
Answer:
The answer is option BExplanation:
The density of a substance can be found by using the formula
\(Density = \frac{mass}{volume} \)
From the question
mass of CCl4 = 3.16 g
volume of CCl4 = 2.0 mL
Substitute the values into the above formula and solve for the Density
That's
\(Density = \frac{3.16}{2} \)
We have the final answer as
Density = 1.58 g/mLHope this helps you
Which statement describes all chemical changes, but not all physical changes?
Answers
Answer:
A new substance forms.
Explanation:
Answer:
B
Explanation:
Please help me do this

Answers
The total mass of the balloon and its content is 1521.17 g, the number of moles of CO₂ in the balloon is 34.15 mol, and the number of CO₂ molecules in the balloon is 2.06 x 10²⁵ molecules.
a) The molar mass of CO₂ is 44.01 g/mol. To find the total mass of the balloon and its content, we need to add the mass of the balloon (20g) to the mass of the CO₂ inside the balloon.
Mass of CO₂ = number of moles of CO₂ x molar mass of CO₂
Since the balloon is at STP (standard temperature and pressure), we can use the molar volume of a gas at STP (22.4 L/mol) to find the number of moles of CO₂ in the balloon:
Volume of CO₂ = Volume of balloon = 765 L (at STP)
Number of moles of CO₂ = volume of CO₂ / molar volume of a gas at STP
= 765 L / 22.4 L/mol
= 34.15 mol
Mass of CO₂ = 34.15 mol x 44.01 g/mol
= 1501.17 g
Total mass of balloon and its content = 20 g + 1501.17 g
= 1521.17 g
b) Number of moles of CO₂ in the balloon is 34.15 mol
c) To find the number of CO₂ molecules in the balloon, we need to use Avogadro's number (6.02 x 10²³ molecules/mol).
Number of CO₂ molecules = number of moles of CO₂ x Avogadro's number
= 34.15 mol x 6.02 x 10²³ molecules/mol
= 2.06 x 10²⁵ molecules
To know more about the Mass, here
https://brainly.com/question/22104139
#SPJ1
Help!!!
A. A halogen
B. An alkaline earth metal
C. An alkali metal
D. A transition element

Answers
Answer:
B?
Explanation:
Write the empirical formula of the compound with the following composition.
63.15% C
5.30% H
31.55% O
Answers
Answer:
C₈H₈O₃
Explanation:
The empirical formula is the simplest whole-number ratio of atoms in a compound.
The ratio of atoms is the same as the ratio of moles.
So, our job is to calculate the molar ratio of C:H:O.
Assume 100 g of the compound.
1. Calculate the mass of each element.
Then we have 63.15 g C, 5.30 g H, and 31.55 g O.
2. Calculate the moles of each element
\(\text{Moles of C} = \text{63.15 g C} \times \dfrac{\text{1 mol C}}{\text{12.01 g C}} = \text{5.258 mol C}\\\\\text{Moles of H} = \text{5.30 g H} \times \dfrac{\text{1 mol H}}{\text{1.008 g H }} = \text{5.258 mol H}\\\\\text{Moles of O} = \text{31.55 g O} \times \dfrac{\text{1 mol O}}{\text{16.00 g O }} = \text{1.972 mol O}\)
3. Calculate the molar ratio of the elements
Divide each number by the smallest number of moles
C:H:O = 5.258:5.258:1.972 = 2.667:2.666:1
4. Multiply by a number to make each ratio close to an integer
Multiply the ratios by three.
2.667:2.666:1 = 8.000:8.000:3 ≈ 8:8:3
5. Write the empirical formula
EF = C₈H₈O₃
It requires two sticks of butter to make a batch of 20 cookies. How much butter will it take to make 150 cookies?
Answers
Answer:
It would take 15 sticks of butter
Explanation:
2 divided by 20 is 0.1
0.1 is how much butter for 1 so now we multiply that by 150
0.1 x 150 = 15
Therefore, you would need 15 sticks of butter for 150 cookies
If the rock is tossed in the ocean, will it’s mass change?
Answers
Answer:
no
Explanation:
if it hits the ocean it either floats or sinks depends what type of rock your tossing is it flat or is it not???
If a material is ductile, it is mostly likely a
nonmetal
metal
metalloid
Gas
Answers
Answer:
Explanation:
Which of the following is most likely to be ductile?
a. Metal
b. Nonmetal
c. Metalloid
d. Gas
Answer: a. MetalMetal
If the radioactive element you were studying in the simulation were carbon – 14, will a half laugh of 5730 years, how long would it take for the sample to decay completly? Examine table 25.3 in the textbook and write the decay reaction for carbon – 14.
Answers
We could obtain the number of years that it would take to decay completely from the number of half lives that are possible for the sample.
What is the half life?The half life of carbon-14 is the time that it takes for only half of the number of the radioactive atoms to remain. We know that carbon 14 is present in all the living things and its level only begins tp diminish in an organism when the organism dies.
Thus, given that the half life of carbon-14 is 5730 years, we could obtain the number of years that it would take to decay completely from the number of half lives that are possible for the sample.
Learn more about half life:https://brainly.com/question/24710827
#SPJ1
Which part of the manufacturing process occurs when binders are added to a paint mixture?
Answers
Answer:
I know this was 2 weeks ago, but it was combining.
Explanation:
Answer:
Combining
Explanation
Question 1
Given the equation: Q = mcAT
Q = heat (in Joules)
m = mass (in grams)
C = 4.18 (specific heat capacity)
AT change in temperature (°C)
How many Joules of heat energy are absorbed when 200 grams of water are heated from 20 C to 60 C.

Answers
The amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
To find the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C, we can use the equation Q = mcAT.
First, we need to find the value of m, which is the mass of the water in grams. In this case, it is given as 200 grams.
Next, we need to find the value of AT, which is the change in temperature in degrees Celsius.
This can be calculated by subtracting the initial temperature from the final temperature, which gives us 60 C - 20 C = 40 C.
The specific heat capacity of water, C, is given as 4.18 Joules per gram per degree Celsius.
Now we can plug in the values into the equation:
Q = mcAT
Q = (200 g) x (4.18 J/g°C) x (40°C)
Q = 33,440 J
Therefore, the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
for more such question on heat energy
https://brainly.com/question/25603269
#SPJ8
whats the awnser? i cant find it and i need it asap! anyone i will crown you the brainleist!
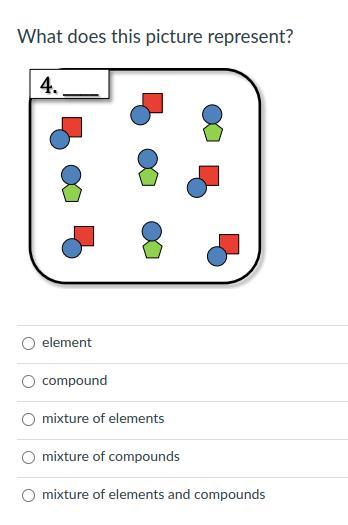
Answers
what is the distinction between magma and lava
Answers
Answer:
Scientists use the term magma for molten rock that is underground and lava for molten rock that breaks through the Earth's surface.
5. The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures
Answers
There are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
To determine the number of water molecules in the given volume of water, we need to use the relationship between mass, volume, and molar mass of water.
First, we need to find the mass of water in the bottle:
Mass = Density * Volume
Mass = 0.967 g/mL * 499.8 mL = 483.9 g
Next, we need to convert the mass of water to moles using the molar mass of water. The molar mass of water (H2O) is approximately 18.015 g/mol.
Moles = Mass / Molar mass
Moles = 483.9 g / 18.015 g/mol = 26.88 mol
Finally, we can calculate the number of water molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules = Moles * Avogadro's number
Number of molecules = 26.88 mol * (6.022 x 10^23 molecules/mol) = 1.62 x 10^25 molecules
Therefore, there are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ8
if a piece of alumuiniam(Al) fail measuring 24cm by 31cm has a mass of 10.35g(density of Al=2.70gcm-3)find the thickness of the fail in millimater?
Answers
Thickness of the fail in millimeter is 0.051mm
Thickness is the quality or state of being thick
Here given data is
aluminum(Al) = 24cm by 31cm
Mass =10.35g
Density = 2.70gcm³
We have to find the thickness of the fail in millimeter = ?
So the formula is
thickness = Volume/surface area
So first we have to calculate volume
Volume = mass/density
Volume = 10.35g/ 2.70gcm³
Volume = 3.83
Then the area of foil is
Area = 24cm×31cm
Area = 744cm²
Thickness = volume/area
Thickness = 3.83/744cm²
Thickness = 0.0051cm = 0.051mm
Thickness of the fail in millimeter is 0.051mm
Know more about aluminum(Al) foil
https://brainly.com/question/17248652
#SPJ9
What's galactose's empirical formula?
Answers
Answer:
C6H12O6
Explanation:
C6H12O6
A certain bimolecular reaction at 40 °C at an activation energy of 30 kJ/mol. The addition of a catalyst reduces the activation energy by a factor of 2. How much faster does the catalyzed occur?
Select one:
OA. 318.63
OB. 358.63
C. 338.63
OD. 378.63
Answers
k = Ae^(-Ea/RT)
where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant (8.314 J/mol·K), and T is the temperature in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin:
40°C + 273.15 = 313.15 K
Now, let's find the ratio of the rate constants for the catalyzed and uncatalyzed reactions:
k_catalyzed / k_uncatalyzed = e^((Ea_uncatalyzed - Ea_catalyzed) / RT)
Since the catalyst reduces the activation energy by a factor of 2:
Ea_catalyzed = 30 kJ/mol / 2 = 15 kJ/mol
Convert the activation energies to J/mol:
Ea_uncatalyzed = 30,000 J/mol
Ea_catalyzed = 15,000 J/mol
Now, plug in the values:
k_catalyzed / k_uncatalyzed = e^((30,000 J/mol - 15,000 J/mol) / (8.314 J/mol·K × 313.15 K))
k_catalyzed / k_uncatalyzed ≈ 358.63
The catalyzed reaction occurs approximately 358.63 times faster. So, the correct answer is OB. 358.63.
What is the mass of the polypropylene in grams? Assume that 1 lbs = 453.952 grams. Drag and drop the
correct conversion factor that will allow you to convert to grams properly.

Answers
Answer:
7.50×10¯² lbs = 7.50×10¯² lbs × 453.952 g / 1 lbs
7.50×10¯² lbs = 34.0464 g.
Explanation:
From the question given, the following data were obtained:
1 lbs = 453.952 g
7.50×10¯² lbs =.?
Thus, we can obtain convert 7.50×10¯² lbs to grams as follow:
1 lbs = 453.952 g
Therefore,
7.50×10¯² lbs = 7.50×10¯² lbs × 453.952 g / 1 lbs
7.50×10¯² lbs = 34.0464 g
Therefore, 7.50×10¯² lbs is equivalent to 34.0464 g.
Which is an example of an inherited behavior (instinct)
Answers
Answer:
Inherited behaviors are behaviors that are passed down genetically. Our genes control things like our hair type and color, our eye color, and our height—but we don't usually think of them controlling our behavior
Explanation:
2. In which state of matter do particles vibrate in place? *
1 point
A. gas
B. solid
C. liquid
Answers
solid.................
Answer:
b solid
Explanation:
i know for a fact
which elements are solid at room temperature
Answers
Answer:bromine , neon , helium , argon , lithium , beryllium
Explanation:
Elements can be divided into three state that are solid, liquid and gas depending the intermolecular forces of attraction, distance between the particles etc. Lithium, beryllium, boron, carbon, sodium, magnesium, iron, copper, etc. are solid at room temperature.
What is element?Element generally consist of atoms or we can atoms combine to form element. Atoms of an element is always same, means all the properties of all atoms of one type of element is same.
Two or more than two atoms with different physical or chemical properties can not combine together to form an element. Elements can be solid liquid or gas at room temperature.
Therefore, Lithium, beryllium, boron, carbon, sodium, magnesium, iron, copper, etc. are solid at room temperature.
To know more about element, here:
https://brainly.com/question/8460633
#SPJ2
The gas carbon dioxide is a pure substance. Which of the following is true about carbon dioxide? (5 points)
Select one:
a. Carbon and oxygen are chemically bonded in it.
b. Carbon and oxygen retain their original identity in it.
c. It can be separated into carbon and oxygen using physical methods.
d. The proportion of carbon and oxygen is different in different samples of the gas.
Answers
Answer:
Carbon and oxygen are chemically bonded in it.
Explanation:
The other answer choices do not apply for compounds, but rather for mixtures instead.
If 25 g of Al was added to 90 g of HCl, what mass of H2 will be produced?
Answers
Answer:
im pretty sure its 2.7 gms
Explanation:
im not for sure tho
2Al+6HCl-----2AlCl3+3H2
54gms reacts with HCl to give 6gms
25gms reacts with HCl to give 2.7gms
A 15.0 mL portion of a 0.400 M solution of acetic acid is to be titrated with a standarized 0.250 M solution of KOH. What is the expected volume of the KOH solution needed to reach the phenolphthalein end point?
Answers
b.9.38 ml.The volume of KOH required to neutralize the acetic acid is:9.38ml
The equation for the reaction between acetic acid (CH3COOH) and potassium hydroxide (KOH) is:
CH3COOH + KOH → CH3COO- + K+ + H2O
The titration of acetic acid with potassium hydroxide can be used to determine the concentration of the acetic acid solution. The volume of KOH required to neutralize the acetic acid can be calculated using the equation:
Volume (KOH) =\(\frac{ (Molarity of acetic acid) * (Volume of acetic acid) }{(Molarity of KOH)}\)
In this problem, the volume of KOH required to neutralize the acetic acid is:
Volume (KOH)
\(\frac{(0.400 M) * (15.0 mL) }{ (0.250 M)}\\= 9.38 mL\)
Therefore,It takes 9.38 ml of KOH to neutralise 1 g of acetic acid.
learn more about acetic acid Refer:brainly.com/question/15202177
#SPJ1
Complete question:A 15.0 mL portion of a 0.400 M solution of acetic acid is to be titrated with a standarized 0.250 M solution of KOH. What is the expected volume of the KOH solution needed to reach the phenolphthalein end point?
a.22.50 ml
b.9.38 ml
c.6.00 ml
d.3.75 ml
e.24.00 ml
What types of clothes should we wear in kitchen cotton or silk ?why?
Answers
Answer:
I think cotton
Explanation:
Because in the kitchen ,there is heat that makes us sweat as cotton is a good absorber of water, it will keep us cool in hot atmosphere of the kitchen.
Sorry but please recheck it again this is my analysis
What is an energy transformation?
Describe the concept of “hidden energy” in an energy transformation?
Answers
While energy can be transferred or transformed, the total amount of energy does not change – this is called energy conservation.
Energy transformation, also known as energy conversion, is the process of changing energy from one form to another. In physics, energy is a quantity that provides the capacity to perform work or provides heat.
When energy is transformed from one form to another, or moved from one place to another, or from one system to another there is energy loss. This means that when energy is converted to a different form, some of the input energy is turned into a highly disordered form of energy, like heat. This consent is known as “hidden energy”.
What mass in grams of potassium chloride is produced if 21.0g of potassium metal.?
Answers
78.2g is the mass in grams of potassium chloride is produced if 21.0g of potassium metal.
A body's mass is an inherent quality. Prior to the discoveries of the atom as well as particle physics, it was widely considered to be tied to the amount of matter within a physical body. It was discovered that, despite having the same quantity of matter in theory.
Different atoms and elementary particles have varied masses. There are various conceptions of mass in contemporary physics that are theoretically different but physically equivalent.
2K + Cl\(_2\) →2KCl
moles of K = 21.0/ 20=1.05 moles
according to stoichiometric ratio
moles of KCl = 1.05 moles
mass of KCl = 1.05×74.5
= 78.2g
To know more about mass, here:
https://brainly.com/question/19694949
#SPJ1
The number of protons plus the number of neutrons equal the ______
1: Atomic Weight
2: Atomic Number
Answers
Determine what elements are denoted by the following electron configuration

Answers
The given electron configuration 1s² 2s² 2p⁶ 3s² 3p⁴ denotes the elements helium (He), beryllium (Be), carbon (C), magnesium (Mg), and sulfur (S).
The given electron configuration represents the electron distribution of an atom. Let's break it down and determine the elements denoted by each part:
1s²: This indicates that there are two electrons in the 1s orbital. The first electron shell (n = 1) can hold a maximum of two electrons. Therefore, the first element denoted by this configuration is helium (He).
2s²: This signifies that there are two electrons in the 2s orbital. The second electron shell (n = 2) can hold a maximum of eight electrons. The element with this configuration is beryllium (Be).
2p⁶: This indicates that there are six electrons in the 2p orbitals. The p orbitals are a set of three orbitals within the second electron shell. The element with this configuration is carbon (C).
3s²: This signifies that there are two electrons in the 3s orbital. The third electron shell (n = 3) can also hold a maximum of eight electrons. The element with this configuration is magnesium (Mg).
3p⁴: This indicates that there are four electrons in the 3p orbitals. The p orbitals in the third shell are also a set of three orbitals. The element with this configuration is sulfur (S).
Know more about the electron configuration here:
https://brainly.com/question/26084288
#SPJ8