Answers
Answer:
1. yes
2. yes
3. no
sorry if I am wrong but hope this helps
Related Questions
Calculate the work done when 1.000 mole of a perfect gas expands reversibly from 1.0 L to 10 L at 298.0 K. Then, calculate the amount of work done when the gas expands irreversibly against a constant external pressure of 1.00 atm. Compare the two values and comment.
Answers
Answer:
See explanation
Explanation:
For work done at constant temperature;
w = -nRTln(V2/V1)
V1 = 1.0 L
V2 = 10 L
w = -(1 * 8.314 * 298) ln(10/1)
w = -5704.8 J
When the gas expands irreversibly against a constant external pressure;
w = -(PΔV)
w = -(1.00 (10 - 1))
w = - 9 J
During expansion of a gas in which the initial and final limits of volume are given, the constant pressure process produces more work than the isothermal process as we have seen above.
hydrogen peroxide slowly decomposes into water and oxygen.the enthalpy change of reaction can be calculated using standard enthalpies of formation.Using a Hess cycle what is the enthalpy change of reaction for this decomposition
Answers
Which of the following compounds has only primary and secondary hydrogen atoms? O 2-Methylpentane O 2.2,3-Trimethylpentane О 3-Methylpentane O 2.2-Dimethylpentane
Answers
The chemical supplied in this option, 2,2-Dimethylpentane, includes both the main and secondary hydrogen atoms that are needed.
A hydrogen atom that is found on a secondary carbon in an organic species is known as secondary hydrogen. The six hydrogen atoms that are connected to the secondary carbon atoms (C2, C4, and C6) are secondary hydrogen atoms. Primary hydrogen is hydrogen that is joined to one carbon atom and then to one additional carbon atom. So, only primary hydrogens are present in isobutene in all of the examples given.
Learn more about hydrogen,
https://brainly.com/question/24433860
#SPJ4
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
Which of these four elements is the most reactive
1: Na
2: Al
3: Rb
4: In
Answers
Answer:
1: Na
Explanation:
Out of the four elements, the most reactive element is sodium (Na).
Sodium is a highly reactive metal because it has only one valence electron in its outermost shell, which is relatively far from the positively charged nucleus. This makes it easy for sodium to lose its outermost electron and form a positively charged ion, which is why it readily reacts with other elements.
Aluminum (Al), rubidium (Rb), and indium (In) are also reactive metals, but they are less reactive than sodium.
Please please help please help me please please help me

Answers
Answer: the left group is the alkali metals while in the right side its the halogens... up to you to pick. either A or D
Explanation:
lndicate the ionisation of the following acids,tetraoxosulphate (vi)acid,trioxonitrat
e(v)acid,ethanoic acid.
Answers
The ionization of the following acids can be represented as:
Tetraoxosulphate (VI) Acid (\(H_{2}SO_{4}\)) ionizes as H+ and SO4^2- ions.
Trioxonitrate (V) Acid (\(HNO_{3}\)) ionizes as H+ and \(NO_{3-}\) ions.
Ethanoic Acid (\(CH_{3}COOH\)) ionizes as H+ and \(CH_{3}COO^{-}\) ions.
Tetraoxosulphate (VI) Acid, also known as sulfuric acid (\(H_{2}SO_{4}\)), ionizes as follows:
\(H_{2}SO_{4}\) → \(H+\) + \(SO_{4}^{2-}\)
In this reaction, sulfuric acid donates two hydrogen ions (H+) to the solution, forming sulfate ions (\(SO_{4}^{2-}\)).
Trioxonitrate (V) Acid, commonly known as nitric acid (\(HNO_{3}\)), ionizes as follows:
\(HNO_{3}\) → \(H+_{}\) + \(NO_{3-}\)
Nitric acid dissociates to release one hydrogen ion (\(H+\)) and a nitrate ion (\(NO_{3-}\)).
Ethanoic Acid, also known as acetic acid (\(CH_{3}COOH\)), ionizes as follows:
\(CH_{3}COOH\) → H+ + \(CH_{3}COO^{-}\)
Acetic acid donates a hydrogen ion (H+) to the solution, forming an acetate ion (\(CH_{3}COO^{-}\)).
In all cases, the acids dissociate in water, producing hydrogen ions (H+) as positively charged ions and their corresponding anions. The hydrogen ions are responsible for the acidic properties of these substances, while the anions contribute to the overall charge balance in the solution. The ionization of acids allows them to conduct electricity in aqueous solutions and react with other substances.
The question was incomplete. find the full content below:
Indicate the ionization of the following acids,
Tetraoxosulphate (VI) Acid
Trioxonitrate (V) Acid
Ethanoic Acid.
Know more about ionization here:
https://brainly.com/question/30831422
#SPJ8
Guysss how to explain nuclear chemistry? And define nuclear chemistry ?
Answers
Answer:
How do amoeba respire.
Define Diffusion.
what is the reaction of guanine and adenine
Answers
Complementary Base Pairing. Specific pairs can only go together, so when guanine and adenine pair up, its Complementry Base Pairing :)
Hope this helps! Please correct me if im wrong :)
Boyle's Law states that at a constant temperature, the volume of a gas is inversely proportional
to the pressure of the gas. (V:/V2 = P2/Pa). An unknown gas has an initial pressure of 150 kPa
and a volume of 1 L. If the volume is increased to 1.5 L, what will the pressure be now?
Answers
Answer: 100kPa at constant temperature.
Explanation:boyles law v1/v2 = p2/p1
v1 = 1L, p1 = 150 kPa, v2 = 1.5L
so p2 = p1*v1/v2 = 150*1/1.5 = 100 kPa
Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M
Answers
Answer:
\(= \mathbf{C_3H_6O}\)
Explanation:
From the given information, since the molecular mass of the ion M+ is not given;
Let's assume M+ = 58.0423
So, by applying the 13th rule;
we will need to divide the mass by 13, after dividing it;
The quotient n = no. of carbon; &
The addition of the quotient (n) with the remainder r = no. of hydrogen.
So;
\(\dfrac{58}{13}= 4 \ remainder \ 6\)
So;
\(C_nH_{n+r} = C_4H_{4+6}\)
\(= C_4H_{10}\)
From the given information; we have oxygen present, so since the mass of oxygen = 16, we put oxygen in the molecular formula by removing \(CH_4\). Also, since the mass is an even number then Nitrogen is 0.
So, we have:
\(= \mathbf{C_3H_6O}\)
How many molecules of CO2 are there in 3.55 g of CO2?
Answers
Answer:
4.9∗1022 molecules of CO2 in a 3.6 gram sample.
Explanation:
The gram molecular weight of co2 is 44 gms.
So the number of molecules in 44 gms of co2 is 6.023×10^23 .
Then the number of molecules in 3.6 gms of co2 is (6.023×10^23×3.6)÷44 = 4.92×10^22
how many atoms in 0.034 moles of titanium
Answers
Answer:
\( \huge{ \boxed{2.0468 \times {10}^{22} \: \text{atoms} }}\)
Explanation:
To find the number of entities in a given substance we use the formula
\( \bold{N = n \times L} \)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question
n = 0.034 moles
N = 0.034 × 6.02 × 10²³ = 2.0468 × 10²²
We have the final answer as
\( \bold{2.0468 \times {10}^{22} \: \text{atoms}}\)
Part B
Next, you’ll test your hypothesis from part A by examining the reaction times of vinegar and baking soda in water at four different temperatures. You’ll carry out the reaction using water at room temperature (about 25°C), 40°C, 60°C, and 80°C. Make sure that you use the same amounts of vinegar and baking soda for all three three trials.
Gather all the materials, and perform these steps for each trial:
Heat at least
cup (60 milliliters) of water to the required temperature (refer to the data table). Water may be heated on a stove, on a hot plate, or in a microwave oven.
Measure and record the actual temperature of the water.
Measure 1 tablespoon (15 milliliters) of the water into the cup.
Add
teaspoon (1.5 grams) baking soda to the water, and stir until it is dissolved. The solution will be clear.
Measure 1 tablespoon (15 milliliters) of vinegar, but do not pour it into the cup yet.
Very quickly, do all of the following:
a. Pour the measured vinegar into the cup.
b. Start the stopwatch.
c. Stir or carefully swirl the substances in the cup.
The chemical reaction will produce bubbles. You’ll be able to see the bubbles and hear them pop. Watch and listen for when the reaction stops. When it looks and sounds like it has finished, stop the stopwatch.
Record the reaction time in the data table.
Discard the solution down the drain, and rinse the cup.
Repeat steps 1–9 of this procedure, doing three trials for each water temperature. Record the average temperature and reaction time for each set of the three trials. Read this math review to know how to calculate average of a data set.
Answers
The reaction time decreases as the temperature increases of the reaction mixture increases.
A sample record of results is:
Temperature (°C) Trial 1 Trial 2 Trial 3 Average25°C 11 seconds 11 seconds 11 seconds 11 seconds40°C 8 seconds 8 seconds 8 seconds 8 seconds60°C 5 seconds 5 seconds 5 seconds 5 seconds80°C 3 seconds 3 seconds 3 seconds 3 secondsWhat is the effect of an increase in temperature on reaction time?An increase in temperature leads to an increase in reaction rate or a decrease in reaction time.
The increase in temperature provides more thermal energy to the reactant molecules, which leads to an increase in the average kinetic energy of the molecules. As a result, more reactant molecules have sufficient energy to overcome the activation energy barrier and undergo successful collisions, leading to an increased reaction rate.
Learn more about reaction time at: https://brainly.com/question/26142029
#SPJ1
What is the IUPAC name of the following substance?

Answers
The International Union of Pure and Applied Chemistry (IUPAC) provides a standard system for naming organic compounds.
It is essential to learn this nomenclature system to communicate correctly about the chemical structures of compounds and how they relate to each other. Here is the IUPAC name of the following substance.Below is the structure of the given compound: In the given compound, there are four carbon atoms that are connected with single bonds. Carbon atoms are also attached to hydrogen atoms. Since it has four carbons in the main chain, the root name will be "but-". The functional group present in the molecule is the carboxylic acid group (-COOH), which gives the suffix "-oic acid." Therefore, the IUPAC name of the given substance is Butanoic acid.Thus, the IUPAC name of the given compound is Butanoic acid. It is essential to know the IUPAC naming of organic compounds to communicate correctly about the chemical structures of the compounds.
for such more questions on Chemistry
https://brainly.com/question/29886197
#SPJ8
Do you think it might be efficient for an industry to be monopolistically competitive rather than perfectly competitive?defend your answer.
Answers
Answer:
Because a good is always priced higher than its marginal cost, a monopolistically competitive market can never achieve productive or allocative efficiency. ... Because monopolistic firms set prices higher than marginal costs, consumer surplus is significantly less than it would be in a perfectly competitive market.
Explanation:
I think
A monopolistically competitive market can never achieve productive or allocative efficiency since a good is always priced higher than its marginal cost. In monopolistically competitive businesses, suppliers will underproduce.
What is efficiency ?Efficiency is the ability to achieve something or get a desired outcome without wasting resources, time, money, energy, or effort. In a broader sense, it is the capacity to carry out tasks effectively, efficiently, and without wasting time.
Due to the fact that it does not produce at the minimum of its average cost curve or where P = MC, a monopolistically competitive firm is inefficient. As a result, a firm that is monopolistically competitive would typically produce less at a greater cost and charge a higher price than a firm that is perfectly competitive.
Thus, In monopolistically competitive businesses, suppliers will underproduce.
To learn more about efficiency follow the link below;
https://brainly.com/question/13646957
#SPJ2
A student combined two solutions of clear liquids in a test tube, after one minute a solid substance appeared in the test tube. Based on their observations, can the students correctly conclude that a chemical reaction occurred?
Answers
What is the length of the paper clip in cm
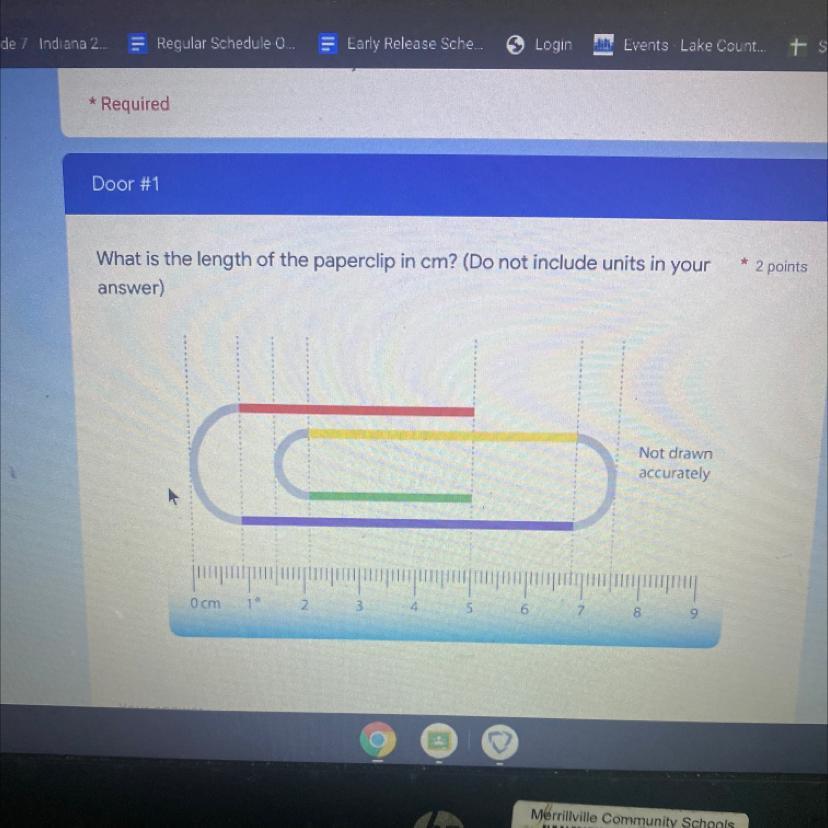
Answers
Answer:
24.5 cm
Explanation:
It would be really complicated to type out, so I've attached an image of how I solved this:
*I separated the paperclip into different sections, figured out the length of those sections, and added them together.
(sorry that my work isn't the neatest)

Which intermolecular force plays a pivotal role in biological molecules such as proteins and DNA ?
•hydrogen bonding
•dispersion force
•dipole-dipole force
•Ion-dipole force
Answers
In the secondary structure of a protein, hydrogen bonds between amino acids determine the configuration of the molecules.
In DNA, hydrogen bonds connect the nitrogenous bases (2 hydrogen bonds between adenine and thymine, 3 hydrogen bonds between guanine and cytosine)
Answer:
hydrogen bonding
Explanation:
just took the test :D

What is the mass of percent of 10 g solute in 105 g solvent?
Answers
The mass of the percent of the 10 g solute in the 105 g solvent is 8.69 %.
The mass of the solute = 10 g
The mass of the solvent = 105 g
The mass of the solution = 10 g + 105 g
The mass of the solution = 115 g
The mass of the percent is expressed as :
The mass of percent = ( mass of solute / mass of solution ) × 100 %
The mass of solute = 10 g
The mass of solution = 115 g
The mass of percent = ( 10 / 115 ) × 100 %
The mass of percent = 8.69 %
Thus, the mass of percent is 8.69 % and the mass of solute is 10 and the mass of the solution is 115 g.
To learn more about mass of percent here
https://brainly.com/question/30742204
#SPJ1
What mass of NaClO2, is contained in 400 grams of water whose
molality is 0.60
Answers
Answer:
Nacio, is contained in 400 grams of water whose molality ... Molality= moles of solute / mass of solvent in Kg Now molality=0.60 Mass
Explanation:
/////
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Calculate the effect of adding H3O+ and OH- on a buffer solution consisting of A) 0.5M CH3COOH and 0.5M CH3COONa B)after adding 0.02 mol of solid NaOH to 1.0L of the buffer solution in part a Ka of CH3COOH= 1.8x10-5 assuming the addition caused negligible volume changes. C) after adding 0.02 mol of HCL to 1.0L of buffer solution in (A).
Answers
A) the addition of \(H_3O\)+ or OH- ions would have opposing effects on the buffer solution.
b) \(H_3O\)+ would favor the formation of acetic acid, while adding OH- would favor the formation of acetate ions.
c) The exact magnitude of the changes in concentrations depends on the initial concentrations of \(CH_3COOH\) and \(CH_3COONa\), as well as the specific amount of \(H_3O\)+ or OH- added.
To determine the effect of adding \(H_3O\)+ and OH- on the given buffer solution, we need to consider the ionization of acetic acid (\(CH_3COOH\)) and the dissociation of its sodium salt (CH3COONa). Let's analyze each scenario separately:
A) Buffer solution consisting of 0.5 M\(CH_3COOH\) and 0.5 M \(CH_3COONa\):
When acetic acid (CH3COOH) and its sodium salt (\(CH_3COONa\)) are present together in a solution, they form a buffer system. Acetic acid partially ionizes in water, releasing \(H_3O\)+ ions, while sodium acetate dissociates into Na+ and \(CH_3COO\)- ions.
Adding \(H_3O\)+:
The \(H_3O\)+ ions would react with the acetate ions (CH3COO-) to form undissociated acetic acid (\(CH_3COOH\)) through the following reaction:
\(H_3O\)+ + \(CH_3COO\)- ⇌ \(CH_3COOH\) + H2O
The addition of H3O+ would shift the equilibrium to the left, promoting the formation of more acetic acid and decreasing the concentration of acetate ions.
Adding OH-:
The OH- ions would react with the acetic acid (\(CH_3COOH\) to form water and acetate ions (CH3COO-) through the following reaction:
OH- + \(CH_3COOH\) ⇌ \(CH_3COO\)- + H2O
The addition of OH- would shift the equilibrium to the right, consuming acetic acid and increasing the concentration of acetate ions.
B) After adding 0.02 mol of NaOH to 1.0 L of the buffer solution:
When solid NaOH is added to the buffer solution, it dissociates completely in water to form Na+ and OH- ions.
NaOH dissociation:
NaOH → Na+ + OH-
The OH- ions formed would react with acetic acid according to the reaction mentioned in Scenario A (2), increasing the concentration of acetate ions and consuming acetic acid.
C) After adding 0.02 mol of HCl to 1.0 L of the buffer solution:
When HCl is added to the buffer solution, it dissociates completely in water to form \(H_3O\)+ and Cl- ions.
HCl dissociation:
HCl → \(H_3O\)+ + Cl-
The \(H_3O\)+ ions formed would react with acetate ions (\(CH_3COO\)-) according to the reaction mentioned in Scenario A (1), forming more undissociated acetic acid and decreasing the concentration of acetate ions.
For more such questions on buffer solution visit:
https://brainly.com/question/13076037
#SPJ8
Fill in the missing information

Answers
Mg^2+- cation, protons: 12, electrons: 10
Te^2- anion, electrons: 8, protons: 52
a 30.00 mL sample of NaOH solution with unknown molarity is neutralized by 35.00 mL of a 0.400 M H2SO4 solution. Determine the unknown molarity.The balanced chemical equation is H2SO4 + 2 NaOH → 2 H2O + Na2SO4
Answers
ANSWER
The molarity of NaOH is 0.933 M
EXPLANATION
Given that;
The volume of NaOH is 30.00mL
The volume of H2SO4 is 35.00mL
The molarity of H2SO4 is 0.400 M
The number of moles of the acid (nA) = 1
The number of moles of the base(nB) = 2
The balanced equation of the reaction is
\(\text{ H}_2SO_4\text{ + 2NaOH }\rightarrow\text{ Na}_2SO_4\text{ + 2H}_2O\)Follow the steps below to find the molarity of NaOH
Step 1; Apply the dilution formula
\(\text{ }\frac{\text{ C}_{A\text{ }}V_A}{\text{ C}_B\text{ V}_B}\text{ = }\frac{\text{ n}_A}{\text{ n}_B}\)\(\begin{gathered} \text{ }\frac{0.4\text{ }\times\text{ 35}}{\text{ C}_B\times\text{ 30}}\text{ = }\frac{\text{ 1}}{\text{ 2}} \\ \text{ cross multiply} \\ \text{ 0.4 }\times\text{ 35 }\times\text{ 2 = C}_B\times\text{ 30 }\times\text{ 1} \\ \text{ 28 = 30C}_B \\ \text{ Divide both sides by 30} \\ \text{ C}_{B\text{ }}\text{ = }\frac{\text{ 28}}{\text{ 30}} \\ \text{ C}_{B\text{ }}\text{ = 0.933 M} \end{gathered}\)Therefore, the molarity of NaOH is 0.933 M
exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. what is the specific heat capacity of the metal
Answers
Exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. The specific heat capacity of the metal is 5.984 J/g°C.
What is specific heat capacity?The heat capacity of a sample of a substance divided by the mass of the sample yields the specific heat capacity (symbol c), also known as massic heat capacity. Informally, it is the quantity of heat that must be added to one unit of a substance's mass in order to raise its temperature by one unit. The specific heat capacity unit in the SI is the joule per kelvin per kilogram, or Jkg⁻¹K⁻¹. For instance, the specific heat capacity of water is 4184 J kg⁻¹K⁻¹, or the amount of energy needed to raise 1 kilogram of water by 1 K.
The specific heat capacity of the metal can be calculated using the equation Q = m × c ×ΔT.
Q = 149.6J
m = 10.0g
ΔT = (final Temperature - initial Temperature) = (25°C - 0°C) = 25°C
Plugging these values into the equation, we get:
149.6J = 10.0g × c ×25°C
Solving for c, we get:
c = \(\frac{149.6J}{(10.0g *25C)}\)
c = 5.984 J/g°C
Therefore, the specific heat capacity of the metal is 5.984 J/g°C.
To know more about specific heat capacity, visit:
https://brainly.com/question/29766819
#SPJ1
What is the function of the magnet in NMR spectroscopy?
Answers
The function of the magnet in Nuclear Magnetic Resonance (NMR) spectroscopy is to generate a strong, uniform magnetic field that aligns the nuclear spins of the sample being analyzed. This alignment allows for the detection and measurement of the interaction between the nuclear spins and an applied radiofrequency pulse.
In NMR spectroscopy, the magnet creates a static magnetic field (B0), which causes the nuclear spins of atoms with a magnetic moment, such as 1H and 13C, to align either parallel or anti-parallel to the field. The energy difference between these two alignments is proportional to the strength of the magnetic field, and results in distinct resonance frequencies for each nucleus.
When a radiofrequency (RF) pulse is applied at the appropriate frequency, it excites the nuclear spins, causing them to flip between the two energy states. After the pulse, the spins return to their equilibrium state, and the process of relaxation emits a detectable signal. This signal, called Free Induction Decay (FID), contains information about the chemical environment of the nuclei, which can be analyzed to provide insights into molecular structure and dynamics.
The quality and resolution of NMR spectra depend on the uniformity and stability of the magnetic field, which is why high-quality magnets, such as superconducting magnets, are essential in NMR spectroscopy. These magnets provide strong, stable magnetic fields that enable the accurate analysis of complex molecular systems.
Know more about spectroscopy here:
https://brainly.com/question/30507284
#SPJ11
What mass of oxygen is needed to complete the combustion of 8.80 x 10^-3 g of methane?
Answers
Explanation:
The chemical reaction that describes the combustion of methane is
\(\text{CH}_4 + 2\text{O}_2 \rightarrow \text{CO}_2 + 2\text{H}_2\text{O}\)
We need to convert the mass of methane to moles:
\(0.00880\:\text{g} × \dfrac{1\:\text{mol CH}_4}{16.04\:\text{g}}\)
\(= 5.49×10^{-4}\:\text{mol CH}_4\)
Now use the molar ratios to determine the amount of oxygen used during the combustion:
\(5.49×10^{-4}\:\text{mol CH}_4×\left(\dfrac{2\:\text{mol O}_2}{1\:\text{mol CH}_4}\right)\)
\( = 0.00110\:\text{mol O}_2\)
Converting this to grams, we find that the mass of oxygen is
\(0.00110\:\text{mol O}_2×\left(\dfrac{15.999\:\text{g O}_2}{1\:\text{mol O}_2}\right)\)
\( = 0.0176\:\text{g O}_2\)
What fraction of a 100 g sample of K - 42 will remain after 24.8 hours?
Answers
Answer:
1/4
Explanation:
From the question given above, the following data were:
Original amount (N₀) = 100 g
Time (t) = 24.8 h
Fraction remaining =?
NOTE: The half-life of K–42 is 12.4 h
Next, we shall determine the number of half-lives that has elapse. This can be obtained as follow:
Time (t) = 24.8 h
Half-life (t½) = 12.4 h
Number of half-lives (n) =?
n = t / t½
n = 24.8 / 12.4
n = 2
Finally, we shall determine the fraction remaining. This can be obtained as follow:
Number of half-lives (n) = 2
Fraction remaining =?
Fraction remaining = 1/2ⁿ
Fraction remaining = 1/2²
Fraction remaining = 1/4
A 32.3-gram sample of gas is found to have a volume of 1.9 liters at 301 K and 1.21 atm. What is the molar mass of this gas? Show all of the work used to solve this problem.
Answers
Answer:
351.1g/mol
Explanation:
you can find the answer using The ideal gas equation
n= PV/RT
n=(1.21*1.9/0.082*301)mol
n=0.092 mol
molar mass=Mass/mole
m=32.3g/0.092mol
m=351.1g/mol