Both the Heisenberg uncertainty principle and the Schrödinger wave equation
Selected Answer:
Answers:
a. led to locating an electron in an atom.
b. are based on Bohr's theory.
c. treat electrons as particles.
d. led to the concept of atomic orbitals.
Answers
Both the Heisenberg uncertainty principle and the Schrödinger wave equation led to the concept of atomic orbitals, hence option D is correct.
The Heisenberg uncertainty principle claimed that it was impossible to know an electron's position and velocity at the same time. It gave rise to the notion that an electron would follow an orbital path, along which a general area could be identified.
It is defined as the presumption that a classical ensemble is susceptible to random momentum fluctuations of a strength that is dictated by and scales inversely with uncertainty in position.
Learn more about uncertainty principle, here:
https://brainly.com/question/30402752
#SPJ1
Related Questions
A gas occupies 1200 litres at 2 atm pressure. To what pressure must it be compressed to occupy 60 litres at the same temperature?
Answers
P2 = 40 atm
Explanation:
Given:
P1 = 2 atm
V1 = 1200 L
V2 = 60 L
P2 = ?
Using Boyle's law and solving for P2,
P1V1 = P2V2
P2 = (V1/V2)P1
= (1200 L/60 L)(2 atm)
= 40 atm
Generic Formula & Molecular Geometry of o3
Answers
Answer:
We use the following formula as given below Use the formula below to find the lone pair on the oxygen atom of the SO3 molecule. L.P (O) = V.E (O) – N.A (S-O) Lone pair on the terminal oxygen atom in SO3 = L.P (O)
Explanation:
a- What is the balanced equation for the reaction of aluminum metal with liquid bromine to produce aluminum bromide?b- How many atoms of aluminum are present initially?c- How many MOLECULES of bromine (Br2) are present initially?d- How many molecules of aluminum bromide (AlBr3) will be produced?e- Which reactant, aluminum or bromine, is the limiting reactant?f- Which reactant, aluminum or bromine, is the excess reactant?g- How many molecules/atoms of excess reactant will remain after the reaction is complete?

Answers
a- Aluminium bromide has the following formula: AlBr₃, so the unbalanced equation is:
\(Al+Br_2\to AlBr_3\)As we can see, for now the aluminium atoms are balanced, but the bromine is not. To balance the bromine, we can put 3 in front of Br₂ and 2 in front of AlBr₃. That way, we will have a total of 6 bromine atoms in each side:
\(Al+3Br_2\to2AlBr_3\)But now the Al is unbalaced, so to fix it we can add a 2 in front of Al to get the balanced equation:
\(2Al+3Br_2\to2AlBr_3\)b- The aluminium are the lone atoms, so, counting them, we see that there are 8 atoms initially.
c- Each pair of empty circles represent a molecule of Br₂, counting them we have 6 molecules initially.
d- The proportion of Al to AlBr₃ is 2:2, that is, 1:1, so if all Al reacts, we would produce the same amount of AlBr₃ as Al, which would be 8 molecules.
The proportion of Br₂ to AlBr₃ is 3:2, so is all Br₂ reacts we will get 2/3 of that as AlBr₃, which would be 6*2/3 = 4 molecules.
This shows that there is not enough Br₂ to react with all 8 atoms of Al, meaning only 4 molecules of AlBr₃ will be produced.
e- Since there is not enough Br₂ to react with all Al present, the limiting reactant is the bromine.
f- The excess reactant is the other one, so if bromine is the limiting, the aluminium is the excess reactant.
g- Since only 4 molecules of AlBr₃ will be formed with all the bromine present, since the proportion of Al to AlBr₃ is 1:1, we wil need only 4 atoms of Al to produce them, which meand that, from the total 8 atoms, we will get
\(8-4=4\)4 atoms of Al as excess reactant after the reaction is complete.
When is a rock considered an ore?
Check all that apply.
-when it occurs in sufficient amounts to be used to build roads and buildings
-only when it contains lead
-when it contains at least one metallic mineral in sufficient amounts to be extracted profitably
-only when it contains iron
-when it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably
Answers
The correct statements are that a rock is considered an ore when it contains at least one metallic mineral in sufficient amounts to be extracted profitably and when it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably.
When it contains at least one metallic mineral in sufficient amounts to be extracted profitably.
When it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably.
Step-by-step explanation:
When it occurs in sufficient amounts to be used to build roads and buildings: This statement is incorrect. Rocks that are used for construction purposes, such as limestone or sandstone, are not considered ores unless they also contain economically valuable minerals.
Only when it contains lead: This statement is incorrect. Ores are not limited to containing only lead. Ores can contain various metallic minerals, not just lead.
When it contains at least one metallic mineral in sufficient amounts to be extracted profitably: This statement is correct. Ores are rocks that contain valuable metallic minerals in concentrations that make extraction economically feasible. These minerals can include gold, silver, copper, aluminum, zinc, and many others.
Only when it contains iron: This statement is incorrect. While iron ores are commonly known and widely used, ores are not limited to containing only iron. There are numerous other metallic minerals that can be extracted profitably from rocks.
When it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably: This statement is correct. Fluorite and sulfur are examples of non-metallic minerals that can be extracted profitably from rocks, and if a rock contains these minerals in sufficient quantities, it can be considered an ore.
learn more about from metallic mineral the given link:
https://brainly.com/question/89259
#SPJ11
As a pollutant such as methylmercury works its way through the food chain, _______ occurs, resulting in top-level food chain members ingesting even higher concentrations of the pollutant.
Answers
As a pollutant such as methylmercury works its way through the food chain, Biomagnification occurs, resulting in top-level food chain members ingesting even higher concentrations of the pollutant.
Biomagnification is the process by which pollutants such as methylmercury become increasingly concentrated as they move up the food chain. This occurs because organisms at lower levels of the food chain ingest small amounts of the pollutant, which then accumulates in their bodies. As larger organisms consume these smaller organisms, they take in a higher concentration of the pollutant. This process continues as the pollutant moves up the food chain, resulting in top-level predators ingesting even higher concentrations of the pollutant. Biomagnification can have serious consequences for the health of these top-level predators, as well as for human populations that consume them.
learn more about Biomagnification
https://brainly.com/question/7631542
#SPJ11
Calculate the mass of NaOH required to prepare a. 459 M solution dissolved in 975 mL of water
Answers
To prepare a 459 M solution of NaOH dissolved in 975 mL of water, we would need 17.901 kg of NaOH.
Molarity is defined as the number of moles of solute per liter of solution. We are given the molarity of the solution as 459 M, which means that there are 459 moles of NaOH per liter of solution.
To find the number of moles of NaOH required to prepare 975 mL of the solution, we need to convert the volume to liters:
975 mL = 975/1000 L = 0.975 L
moles of NaOH = molarity x volume of solution in liters
moles of NaOH = 459 M x 0.975 L = 447.525 moles
mass of NaOH = moles of NaOH x molar mass of NaOH
The molar mass of NaOH is 40.00 g/mol. Therefore:
mass of NaOH = 447.525 moles x 40.00 g/mol = 17,901 g or 17.901 kg.
learn more about molarity here:
https://brainly.com/question/8732513
#SPJ4
Calculate the molarity 14.1 moles of FeCl3 dissolved in 2350 ml of solution
Answers
We have 14.1 moles of FeCl3, which is the solute, and the volume of our solution is 2.350 liters. So, the molarity of the solution would be (14.1 moles FeCl3)/(2.350 L solution) = 6 moles/L FeCl3 or 6 M FeCl3.
in the early 1960s, radioactive strontium-90 was released during atmospheric testing of nuclear weapons and got into the bones of people alive at the time. if the half-life of strontium-90 is 27 years, what fraction of the strontium-90 absorbed in 1962 remained in people's bones in 1990?
Answers
The fraction of the strontium-90 that remained in people's bones in 1990 can be determined by using the formula:
N(t) = N0 * (1/2)^(t/T)
Where N(t) is the remaining amount of strontium-90 in people's bones at time t, N0 is the initial amount absorbed in 1962, t is the time elapsed (1990 - 1962 = 28 years), and T is the half-life of strontium-90 (27 years).
So, N(1990) = N0 * (1/2)^(28/27) = N0 * (1/2)^1 = N0 * (1/2) = 0.5 * N0
Therefore, the fraction of strontium-90 that remained in people's bones in 1990 is 1/2 or 0.5.
How can strontium be dangerous to the body?Strontium ium is veritably analogous to calcium and can replace calcium in the bone. This disrupts the normal bone structure, leading to cadaverous problems and blights. babies, children, adolescents, and people who don't get enough calcium are at advanced threat of dangerous health goods related to strontium exposure.
Is stontium highly radioactive?Strontium is a soft, argentine metallic element set up in jewels, soil, dust, coal and oil painting. Strontium set up in nature isn't radioactive and is occasionally called stable strontium.
To know more about radioactive, visit here:
https://brainly.com/question/1770619
#SPJ4
Night visin cameras are sensitive to energies around 2.21 x 10-19 J. What wavelength of electromagnetic radiation do they use?
Answers
Answer:
8.99×10^-7m
Explanation:
The wavelength can be calculated using the expression below
E=hcλ
Where E= energy= 2.21 x 10^-19 J.
C= speed of light= 3x10^8 m/s
h= planks constant= 6.626 × 10^-34 m2 kg / s
E=hcλ
λ= E/(hc)
Substitute for the values
λ=( 2.21 x 10^-19 )/(6.626 × 10^-34 × 3x10^8 )
= 8.99×10^-7m
Answer:
Look at the explanation this one is hard
Explanation:
show your work:
E = hc/ λ
E λ = hc
λ = hc/E
λ = 6.626 x 10^-34 x 3 x 10^8 / 2.21 x 10^-19
=
8.994 x 10-7 m or 899.4 nm
800nm – 3mm is the range of wavelengths for infrared waves.
Night vision cameras use infrared waves.
Question 28 (4 points)
Complete the following table:
Substance
pH
POH
[H] M
A
3.45
B
0.26
For the concentration fill in the blank, I used a decimal with a leading zero and two sig figs.
Answers
Answer:
Pls type your question in another manner cos I really cant decipher what you wrote the way you typed this pls
A 4.2 M NaCl solution has 86.2 grams of NaCl dissolved in it. How many liters of solution will
this be?
Answers
Answer:
this is your answer (8358) answer
I think
question 38 17) a catabolic pathway may be which of the following? a) a set of reactions that combine monomers into larger, more energy-rich polymers b) a set of coupled reactions that are endergonic c) a set of reactions that form covalent bonds between molecules to store free energy d) a set of reactions that release energy that can be used to drive cellular work
Answers
A catabolic pathway is a d) a set of reactions that release energy that can be used to drive cellular work
A catabolic pathway involves a series of reactions that break down complex molecules into simpler ones, releasing energy in the process. This energy is then utilized by the cell to perform various tasks. Option d correctly identifies a catabolic pathway as a set of reactions that release energy, which can be used to drive cellular work.
During catabolism, large molecules such as carbohydrates, proteins, and fats are broken down into smaller molecules like glucose, amino acids, and fatty acids.
These molecules are then further degraded through oxidation reactions, releasing energy in the form of ATP (adenosine triphosphate). ATP serves as the primary energy currency in cells, providing energy for various cellular processes.
By breaking down complex molecules and releasing energy, catabolic pathways enable cells to obtain the necessary energy to carry out essential functions like metabolism, growth, and movement. So d is correct option.
For more questions like Catabolic pathway click the link below:
https://brainly.com/question/29603008
#SPJ11
1) pbo+co-->pb+co2 which type of reaction is this?
a) redox reaction
b) oxidation reaction
c) reduction reaction
d) none of the above
Answers
PbO + CO → Pb + CO₂
Oxidation States of the Elements
Reactants Products
Pb +2 0
O -2, -2 -4
C +2 +4
Since the oxidation state/number of Pb decreased, it is being Reduced.
Since the oxidation state/number of C increased, it is being Oxidized.
∴ this is a Redox ReactionWrite the net ionic equation for this precipitation reaction. Include physical states : 2RbOH(aq)+Mg(NO3)2(aq)⟶Mg(OH)2(s)+2RbNO3(aq)2RbOH(aq)+Mg(NO3)2(aq)⟶Mg(OH)2(s)+2RbNO3(aq) net ionic equation:
Answers
the net ionic equation for the precipitation reaction, we'll first break down the compounds into their respective ions, and then eliminate spectator ions: Mg2+(aq) + 2OH-(aq) ⟶ Mg(OH)2(s).
In the given reaction, 2RbOH(aq) reacts with Mg(NO3)2(aq) to form Mg(OH)2(s) and 2RbNO3(aq). The balanced chemical equation for this reaction is: 2RbOH(aq) + Mg(NO3)2(aq) ⟶ Mg(OH)2(s) + 2RbNO3(aq).
To write the net ionic equation, we need to cancel out the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the reaction. The spectator ions in this reaction are Rb+(aq) and NO3-(aq).
The net ionic equation only includes the ions that undergo a chemical change. In this case, Mg2+(aq) and OH-(aq) combine to form a precipitate, Mg(OH)2(s).
Break down the compounds into their respective ions:
- 2RbOH(aq) ⟶ 2Rb⁺(aq) + 2OH⁻(aq)
- Mg(NO3)2(aq) ⟶ Mg²⁺(aq) + 2NO₃⁻(aq)
- Mg(OH)2(s) remains as Mg(OH)₂(s) (since it is a solid)
- 2RbNO3(aq) ⟶ 2Rb⁺(aq) + 2NO₃⁻(aq)
Combine the dissociated ions and eliminate the spectator ions (those that do not change during the reaction). In this case, the spectator ions are Rb⁺(aq) and NO₃⁻(aq). Write the net ionic equation with the remaining ions, including their physical states: RbOH(aq) + Mg²⁺(aq) + 2NO₃⁻(aq) ⟶ Mg(OH)₂(s) + Rb⁺(aq) + NO₃⁻(aq).
To know more about reaction visit:
https://brainly.com/question/30464598
#SPJ11
what is the predicted product of the reaction shown hoch2ch2oh h2so4 mg/ether
Answers
The predicted product of the reaction shown is an ether, specifically methyl ethyl ether.
The reaction involves the dehydration of ethanol (HOCH2CH2OH) in the presence of sulfuric acid (H2SO4) and magnesium (Mg), which acts as a catalyst. The sulfuric acid protonates the hydroxyl group in ethanol, making it a better leaving group. The resulting carbocation then undergoes an elimination reaction with the neighboring hydroxyl group, resulting in the formation of methyl ethyl ether.
This reaction is known as the Williamson ether synthesis.
To know more about chemical visit :-
https://brainly.com/question/29886197
#SPJ11
an herbicide contains only c , h , cl , and n . the complete combustion of a 150.0 mg sample of the herbicide in excess oxygen produced 156.9 ml of co2 and 91.52 ml of h2o vapor at stp. a separate analysis determined the 150.0 mg sample contained 41.36 mg cl . determine the percent composition of the herbicide.
Answers
The percent composition of the herbicide is 44.5% C, 6.27% H, 22.9% Cl, and 26.4% N.
To solve this problem, we will use the information provided to calculate the percent composition of the herbicide.
First, let's calculate the number of moles of CO2 and H2O produced by the combustion of the herbicide. We can use the ideal gas law to do this:
n_CO2 = (156.9 mL) / (22.4 L/mol) * (1 mol CO2 / 1 L) = 7.00 mol CO2
n_H2O = (91.52 mL) / (22.4 L/mol) * (1 mol H2O / 1 L) = 4.08 mol H2O
Next, let's calculate the number of moles of carbon, hydrogen, and nitrogen in the herbicide using the combustion reaction:
C_xH_yCl_zN_w + (x + y/4 - z/2) O2 → x CO2 + (y/2) H2O + z HCl + w NO2
From the balanced equation, we can see that the number of moles of CO2 produced is equal to the number of moles of carbon in the herbicide, and the number of moles of H2O produced is equal to the number of moles of hydrogen in the herbicide.
We can use this information to solve for the number of moles of carbon, hydrogen, and nitrogen in the herbicide:
n_C = 7.00 mol CO2
n_H = 8.16 mol H2O
n_Cl = 41.36 mg / 35.45 g/mol / 0.1500 g = 0.767 mol Cl
Since the herbicide contains no other elements besides C, H, Cl, and N, we can assume that the mass of the herbicide is equal to the sum of the masses of these elements. We can use this information to solve for the mass of the herbicide:
m_Herbicide = m_C + m_H + m_Cl + m_N
m_Herbicide = n_C * 12.01 g/mol + n_H * 1.008 g/mol + n_Cl * 35.45 g/mol + n_N * 14.01 g/mol
We can rearrange this equation to solve for the percent composition of the herbicide:
% C = (n_C * 12.01 g/mol / m_Herbicide) * 100% = 44.5%
% H = (n_H * 1.008 g/mol / m_Herbicide) * 100% = 6.27%
% Cl = (n_Cl * 35.45 g/mol / m_Herbicide) * 100% = 22.9%
% N = ((m_Herbicide - n_C * 12.01 g/mol - n_H * 1.008 g/mol - n_Cl * 35.45 g/mol) / m_Herbicide) * 100% = 26.4%
to know more about herbicides refer here:
https://brainly.com/question/31375814#
#SPJ11
The hydrogen emission spectrum is shown below. What is the energy of the
410 nm emission line? (The speed of light in a vacuum is 3.00 x 108 m/s, and
Planck's constant is 6.626 x 10-34 J.s.)
400
750 pm

Answers
Answer:
C.) 4.85 x 10⁻¹⁹ J
Explanation:
To find the energy, you need to use the following equation:
E = hc / w
In this formula,
-----> E = energy (J)
-----> h = Planck's Constant (6.626 x 10⁻³⁴ J*s)
-----> c = speed of light (3.00 x 10⁸ m/s)
-----> w = wavelength (m)
Once you have converted nanometers to meters, you can plug the given values into the equation and solve.
410 nm 1 m
------------- x ---------------------- = 4.10 x 10⁻⁷ m
1 x 10⁹ nm
E = hc / w
E = (6.626 x 10⁻³⁴ J*s)(3.00 x 10⁸ m/s) / (4.10 x 10⁻⁷ m)
E = 4.85 x 10⁻¹⁹ J
A crab has an exoskeleton. The combusting wax in a candle wick is an exothermic reaction. What do you think the prefix “exo” means and how does this apply to the burning wax?
Answers
When the pH of a solution is 2, the concentration of hydronium ions is10-2 M = 0.01 M. Is it acidic or basic?
Answers
Answer:
Explanations:
The formula for calculating the pH of the solution is given as:
\(pH=-log[H_3O^+]\)Given that the pH is 2, then;
\(\begin{gathered} 2=-log[H_3O^+] \\ log[H_3O^+]=-2 \\ [H_3O^+]=10^{-2} \\ [H_3O^+]=0.01M \end{gathered}\)Hence the concentration of the hydronium ion is 0.01M
Since the pH of the solution is less than 7, hence the solution is acidic
Give Me a simple definition for Acids.
Answers
Answer:
an acid is a substance which produces hydrogen ions as the only as the only positive ion when dissolved in water.
What is the molarity of a solution prepared by placing 154.2 g of ammonium nitrate into a 250.00 ml volumetric flask and diluting to the mark with deionized water?
Answers
The molarity of the solution is 7.704 M.
What is Molarity?In terms of the amount of material per unit volume of solution, molar concentration is a measurement of the concentration of a chemical species, specifically a solute in a solution. The most frequent measure of molarity in chemistry is the number of moles per liter, denoted by the unit symbol mol/L or mol/dm3 in SI units.Calculation of molarity:Given,
Mass of solute (ammonium nitrate) = 154.2 g
Volume of solution = 250 ml = 0.25 L
Molar mass of ammonium nitrate = 80.043 g/mol
Number of moles of solute = 154.2/80.043
= 1.926 moles
Molarity = number of moles of solute/ volume of solution
= 1.926/0.25
=7.704 M
Hence, the molarity of the solution is 7.704 M.
Learn more about Molarity here:
https://brainly.com/question/2817451
#SPJ4
Help i’m taking a test!!
What new element is formed from the equation below? (Answer form is
MN:, AN: , S)

Answers
Answer:
Element Z
Explanation:
I hope this is correct and helps you!
Calculate the pH of a solution obtained by mixing 477 mL of 0.17 M hydrochloric acid with 253 mL of M lithium hydroxide. Assume the combined volume is the sum of the two original volumes.
Answers
Answer:
pH = 0.984
Explanation:
Molarity LiOH = 2.1x10⁻²M
HCl will react with LiOH as follows:
HCl + LiOH → H₂O + LiCl
1 moles of HCl reacts per mole of LiOH
Moles of each reactant in solution are:
HCl = 0.477L ₓ (0.17mol / L) = 0.08109 moles HCl
LiOH = 0.253L ₓ (2.1x10⁻² mol / L) = 5.313x10⁻³ moles of LiOH.
That means LiOH is the limiting reactant and excess moles of HCl that will remain in solution are:
0.08109 mol - 0.005313mol = 0.0758 moles HCl
As HCl dissociates in water as H⁺ and Cl⁻ ions, you will have in solution 0.0758 moles of H⁺
pH = -log [H⁺] and [H⁺] = moles H⁺ / L of solution.
Volume of the mixture in liters is: 0.477L + 0.253L = 0.730L.
That means [H⁺] is 0.0758 moles of H⁺ / 0.730L = 0.1038M
Replacing:
pH = -log [H⁺]
pH = -log [0.1038]
pH = 0.984hydrogen molecules (molar mass is equal to 2.016 g/mol) have an average velocity vrms equal to 243 m/s. what is the temperature (in k)?
Answers
The temperature (in k) is 469.4 .The average speed of hydrogen molecules at typical conditions of temperature and pressure is 1.70 103 ms1.
How quickly do hydrogen molecules move on average?The average speed of hydrogen molecules at typical conditions of temperature and pressure is 1.70 103 ms1.An RMS speed of 193 meters per second is the average speed of hydrogen atoms having a molar mass of 2.016 kg per mole.While the formula to convert Kelvins into Celsius degrees is K 273.15 = °C, the formula to convert Kelvins into Fahrenheit degrees is (K 273.15) 9/5 + 32 = °F. A measurement of an object's hotness or coolness can be used to determine temperature.F = C(9/5) + 32 when converting from Celsius to Fahrenheit. From Fahrenheit to Kelvin, K equals (F-32) (5/9) plus 273.15. F = (K-273.15) (9/5) + 32 to convert from Kelvin to Fahrenheit.Explanation:
F = 243(9/5) + 32
= 437.4 + 32
= 469.4.
The temperature (in k) is 469.4 .
To learn more about Hydrogen molecules refer to:
https://brainly.com/question/24317372
#SPJ4
Identify the correct mole ratio for each substance.
Sodium chloride (NaCl)
Na:Cl = 1: _
Ammonium nitrate(NH.NO3)
H:0 = 4: _
Answers
Answer:
The mole ratio of H : O in ammonium nitrate is 4 : 3.
Explanation:
We are given a compound named ammonium nitrate having formula
There are 3 elements in this compound which are nitrogen, hydrogen and oxygen.
To calculate the mole ratio, we write the ratio of their subscripts. For this compound,
An airplane travels 200 km/h in 4 hours going to Zambales. What will be the distance and displacement?
Answers
Answer:
See explanation
Explanation:
Recall that;
Speed = Distance/time
Distance = Speed * time
Speed = 200 km/h
Time = 4 hours
Distance = 200 km/h * 4 hours = 800 kilometres
Displacement has to do with distance covered in a specified direction, in this case, the direction is towards Zambales.
Hence, the displacement is 800 kilometres towards Zambales.
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
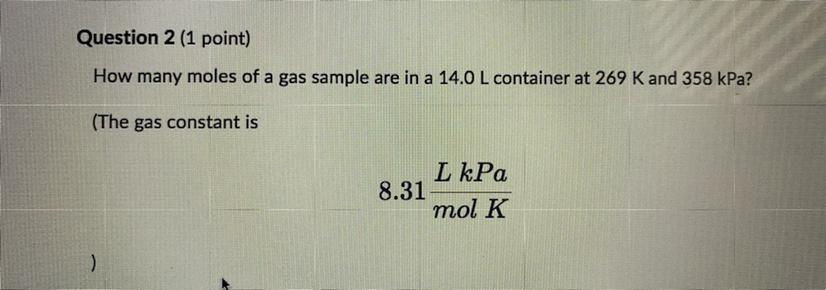
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
An ion that consists of 7 protons, 6 neutrons, and 10 electrons has a net charge of
1.
4-
2.
3-
3.
3+
4.
4+
Answers
Answer: 3+
Explanation:
O=H-H
is an acid,
a base,
Or
neither an
acid nor a
base.

Answers
The given structure is of formaldehyde an organic compound and it is acidic in nature.
Why is acidic formaldehyde?The formic acid is transformed into formaldehyde when hydrogen is added. Because of this, ambient oxygen can more quickly convert formaldehyde into formic acid. In addition to most polar organic solvents, formic acid is miscible with water. Although formaldehyde is a weak acid (pK greater than 13), there was no reliable method to estimate and correct the base bound by formaldehyde because the base bound by wool was always identified by comparing the base present at equilibrium in aliquots of solutions that were identical except for the presence of wool in one of them.Formaldehyde is a combustible, colorless gas that is noticeable for its strong aroma when it is at ambient temperature. Oxomethane, methylaldehyde, oxymethyline, and methanal are some of its other names.For more information on formaldehyde kindly visit to
https://brainly.com/question/29550668
#SPJ1