8. Of what importance is a smoker to a person keeping bees?
Answers
Answer:
they help to calm the bees when the person keeping the bees inspects there hives.
Related Questions
calculate the concentration of a solution containing 1.8g of sodium carbonate in 862cm3 of water
Answers
Answer:
0.02 M
Explanation:
Given data:
Mass of sodium carbonate = 1.8 g
Volume of water = 862 cm³ (862/1000 = 0.862 L)
Concentration = ?
Solution:
Formula:
Concentration = number of moles / volume in L
Number of moles of sodium carbonate:
Number of moles = mass/molar mass
Number of moles = 1.8 g/ 106 g/mol
Number of moles = 0.02 mol
Concentration:
c = 0.02 mol / 0.862 L
c = 0.02 M
Sulfuric acid is produced by first burning sulfur to produce sulfur trioxide gas
2S(s) + 3O2(g) → 2SO3(g)
then dissolving the sulfur trioxide gas in water
SO3(g) + H2O(l) → H2SO4(l)
Calculate the mass of sulfuric acid produced if 1.25 g of sulfur is reacted as indicated in the above equations.
Answers
The mass of sulfuric acid produced is 2.77 g.
To calculate the mass of sulfuric acid produced, we need to use stoichiometry. Here are the step-by-step calculations:
2S(s) + 3O2(g) → 2SO3(g)
SO3(g) + H2O(l) → H2SO4(l)
Calculate the molar mass of sulfur;
S = 32.06 g/mol
Convert the given mass of sulfur to moles;
1.25 g / 32.06 g/mol = 0.039 moles S
Use the mole ratio from the balanced equation to find the moles of sulfur trioxide produced;
0.039 moles S x (2 moles SO3 / 2 moles S) = 0.039 moles SO3
Use the mole ratio from the balanced equation to find the moles of sulfuric acid produced;
0.039 moles SO3 x (1 mole H2SO4 / 1 mole SO3) = 0.039 moles H2SO4
Calculate the mass of sulfuric acid produced;
0.039 moles H2SO4 x 98.08 g/mol = 2.77 g H2SO4
So the mass of sulfuric acid produced is 2.77 g.
Learn more about sulfuric acid brainly.com/question/10220770
#SPJ1
Use these two constants for the question that follows:
• e = 1.6 × 10^-19 C
• k = 8.99 × 10^9 N m^2/C^2
Two negative charges are 10^-14 m away from each other. Using Coulomb's law, which of the following is the electrical force between these two particles?
A) 2.3 N
B) -2.3 N
C) 1.4 N
D) -1.4 N

Answers
The electrical force between these two particles is approximately -2.3 N.
So,the correct answer is (B) -2.3 N
Coulomb’s law is given as:F=ke * q1 * q2 / d^2
Where,F = Force in Newton (N)q1 = Charge on particle 1 in Coulombs (C)q2 = Charge on particle 2 in Coulombs (C)k = Coulomb’s constant = 8.99 × 10^9 N m^2/C^2d = Distance between two particles in meters (m).
Given:e = 1.6 × 10^-19 Ck = 8.99 × 10^9 N m^2/C^2Distance between two particles, d = 10^-14 mAs given, both particles are negative charges; hence, the force between the two particles will be repulsive.
The formula to find the electric force between two negative charges is:F = -ke * q1 * q2 / d^2F = - (8.99 × 10^9 N m^2/C^2) * (1.6 × 10^-19 C) * (1.6 × 10^-19 C) / (10^-14 m)^2= - 2.304 × 10^-9 N= - 2.3 N (approximately).
For more such questions on force
https://brainly.com/question/8106035
#SPJ8
1) an acid base titration involves a a) composition reation b) neutralization reaction c) single-replacement reaction d) decomposition reaction
Answers
An acid-base titration involves a neutralization reaction
An acid-base titration is a laboratory technique that is used to determine the concentration of an acid or a base in a solution. The basic principle behind this technique is the fact that acid-base reactions are reversible, meaning that an acid and a base can react with each other to form a neutral compound.
In an acid-base titration, a known concentration of a base is added to a solution containing an unknown concentration of an acid. The acid and the base react with each other to form a neutral compound, such as water. By carefully measuring the volume of the titrant that is required to neutralize the acid, the concentration of the acid in the solution can be determined. This process is known as a neutralization reaction.
Read more about neutralization reaction on:
https://brainly.com/question/23008798
#SPJ4
An object has a mass of 441 g and a volume of 10cm3
Answers
Answer:
44.1g/cm3
Explanation:
d=m/v
=441/10
=44.1 g/cm3
What is the molar ratio of HBr and KBrO3 you will be adding to this reaction? What molar ratio of HBr and KBrO3 should be used to generate Br2? Consider equation 1 below and answer assuming HBr is the only source of protons. answer question above
Answers
The molar ratio of HBr to KBrO₃ is 3:1 in the balanced chemical equation for the reaction between them. To generate Br₂ using only HBr as the source of protons, the molar ratio of HBr to H₂O₂ is 2:1.
The balanced chemical equation for the reaction between HBr and KBrO₃ is:
3HBr + KBrO₃ → 3Br₂ + KBr + 3H₂O
From the equation, the molar ratio of HBr to KBrO₃ is 3:1. This means that for every 3 moles of HBr used in the reaction, 1 mole of KBrO₃ is needed.
To generate Br₂ using only HBr as the source of protons, the following reaction can be used:
2HBr + H₂O₂ → Br₂ + 2H₂O
The molar ratio of HBr to H₂O₂ in this reaction is 2:1. This means that for every 2 moles of HBr used, 1 mole of H₂O₂ is needed. The molar ratio of HBr and KBrO₃ is not relevant to this reaction since KBrO₃ is not involved.
To know more about the molar ratio refer here :
https://brainly.com/question/17920577#
#SPJ11
How did ethanol use affect the shortage of corn available to consumers during and after the drought of 2012?.
Answers
Ethanol use was effect the shortage of corn available on those time because of corn is an ingredient to produce the ethanol. Hence, ethanol use was aligned with the rarity of corn availability on those time. the more ethanol was used, the more rare corn availability for consumer.
What is the effect of the drought?The drought is a condition where the area cant provide enough water supply for a long. Agriculture sector is the most affected sector when the drought occur. The drought cause crop failure, and this condition create domino effect on another sector such as food price get more expensive, and because of food get more expensive, buying power getting low cause small business out of business.
Learn more about drought here
https://brainly.com/question/12686086
#SPJ4
A 0.50-quart jar contains 2.0 pounds of honey. what is the density of the honey in g/ml?
Answers
A 0.50-quart jar contains 2.0 pounds of honey. 1.9 g/ml is the density of the honey.
Population density (in agriculture, forest stand, or plant density) is a measure of population per unit area or, exceptionally, per unit volume. It is a quantity of type number density. It applies to living things in general, mainly humans. It's an important geographical term. Simply put, population density is the number of people living per square kilometer.
Urban populations, especially urban areas, strongly depend on the definition of "urban area" used. It is almost always denser only in the center than when it includes suburban settlements and intervening rural areas, such as agglomerations and metropolitan areas. Area (the latter may include neighboring cities).
Learn more about density here
https://brainly.com/question/1354972
#SPJ4
a student drops a bottle of shampoo because his hands were slippery from the shampoo. other than its slippery, what other tests would confirm which type of compound was in the shampoo
Answers
To confirm the type of compound present in the shampoo, several tests can be conducted. Here are a few possible tests:
pH Test: Shampoos often contain acidic or basic compounds to maintain the desired pH level. Using pH strips or a pH meter, the student can test the pH of the shampoo. Acidic shampoos typically have a pH below 7, while alkaline shampoos have a pH above 7.
Solubility Test: Different compounds have different solubilities in various solvents. The student can try dissolving a small amount of the shampoo in water, alcohol, or oil to observe the solubility. The results can provide insights into the presence of certain compounds such as salts, surfactants, or oils.
Foaming Test: Shampoos often contain surfactants that produce foam when agitated with water. The student can mix a small amount of shampoo with water and vigorously shake it to observe the formation of foam. This test can indicate the presence of surfactants.
Combustion Test: By carefully burning a small amount of shampoo on a non-flammable surface, the student can observe the flame color and odor. Different compounds produce characteristic flame colors and smells upon combustion, which can help identify specific ingredients.
By conducting these tests, the student can gather additional information about the chemical nature of the compound in the shampoo and narrow down the possibilities of its composition.
To know more about compound present click this link-
https://brainly.com/question/18560111
#SPJ11
HELLPPP PLZZ ASAPP!
Which phase transitions can a gas undergo? Select the two correct answers.
- Vaporization
- Freezing
- Deposition
- Melting
- Sublimation
- Condensation
Answers
Answer:
Condensation and Depositon
Explanation:
Condensation is from gas to liquid
Deposition is from gas to solid
There are moles of Carbon if I have 2.25 x 1023 atoms of Carbon? A. 0.19 moles b. 5.38 moles c. 0.37 moles d. 2.67 moles
Answers
Answer:
c. 0.37 moles
Explanation:
To find the number of moles (n) in a certain number of atoms of a substance, we divide the number of atoms by Avogadro number (nA), which is 6.02 × 10²³ atoms.
That is,
n = number of atoms ÷ 6.02 × 10²³
According to this question, there are 2.25 x 10²³ atoms of carbon (C). The number of moles contained in this carbon is as follows:
n = 2.25 x 10²³ ÷ 6.02 × 10²³
n = 2.25/6.02 × 10 (23 - 23)
n = 0.374 × 1
n = 0.374moles
what reaction (oxidation or reduction) occurs at the anode of a voltaic cell?
Answers
The reaction that occurs at the anode of a voltaic cell depends on the specific cell being used.
In general, however, the anode is the electrode where oxidation occurs, meaning that electrons are lost from the anode. This oxidation process is what creates the electrical potential difference that drives the flow of electrons through the cell and produces an electric current. On the other hand, reduction occurs at the cathode, which is the electrode where electrons are gained.
Together, the oxidation and reduction reactions that occur at the anode and cathode of a voltaic cell create a balanced redox reaction, which ultimately produces the flow of electricity that powers the cell.
To know more about reaction visit:-
https://brainly.com/question/30464598
#SPJ11
A lab group was measuring the mass of an object. They recorded the following masses for the same object: 12.13 g, 12.12 g, 12.13 g, and 12.11 g. The actual mass of the object was 17.13 g. Which of the following best describes the lab groups results? The results were both accurate and precise. The results were accurate but not precise. The results were precise but not accurate. The results lacked both precision and accuracy.
Answers
Answer:
Precise, but not accurate
Explanation:
First, let’s define accuracy and precision.
⇒Accuracy: how close you are to the actual value
⇒Precision: how close the measurements are to each other.
Let’s examine the data given:
12.13 g, 12.12 g, 12.13 g, and 12.11 g
Each number is or is very close to 12.12 (within 0.1)
The actual mass of the object is 17.13 grams. Each measurement is about 5 grams away from the actual mass.
Since the measurements are very close to each other, but quite far from the true value, we can say the results are precise, but not accurate.
The solid, liquid, and gas states of water differ from each other in
Ο Α.
the mass of the individual molecules.
O B.
the size of the individual molecules.
Ос.
the average speed of the molecules.
O D.
the electrical charge of the molecules.
Answers
Answer:
B
Explanation:
the size of the individual molecules.
A sample of X occupies 80.0 L at 250°C. Assuming constant pressure, what is the absolute temperature if the volume decreased to 40.0 L?
Answers
Assuming constant pressure, the absolute temperature of the sample is 500 K can be found by Charles's Law,.
When the volume of the gas decreases from 80.0 L to 40.0 L at constant pressure, according to Charles's Law, the temperature must also decrease by half to maintain the constant pressure. Therefore, the new temperature of the gas can be calculated by multiplying the initial temperature (250°C = 523.15 K) by the ratio of final volume to initial volume (40.0/80.0 = 0.5), giving us a temperature of 500 K. It's important to note that the temperature must be expressed in absolute units (Kelvin) when using gas laws, as they are proportional to absolute temperature.
To learn more about Charles's Law, click here:
https://brainly.com/question/16927784
#SPJ11
help me and explain steps

Answers
Answer:
2 Fe + 3 H2SO4 —> Fe2(SO4)3 + 3 H2
I hope I helped you^_^
See photo please!! I only have 10 mins
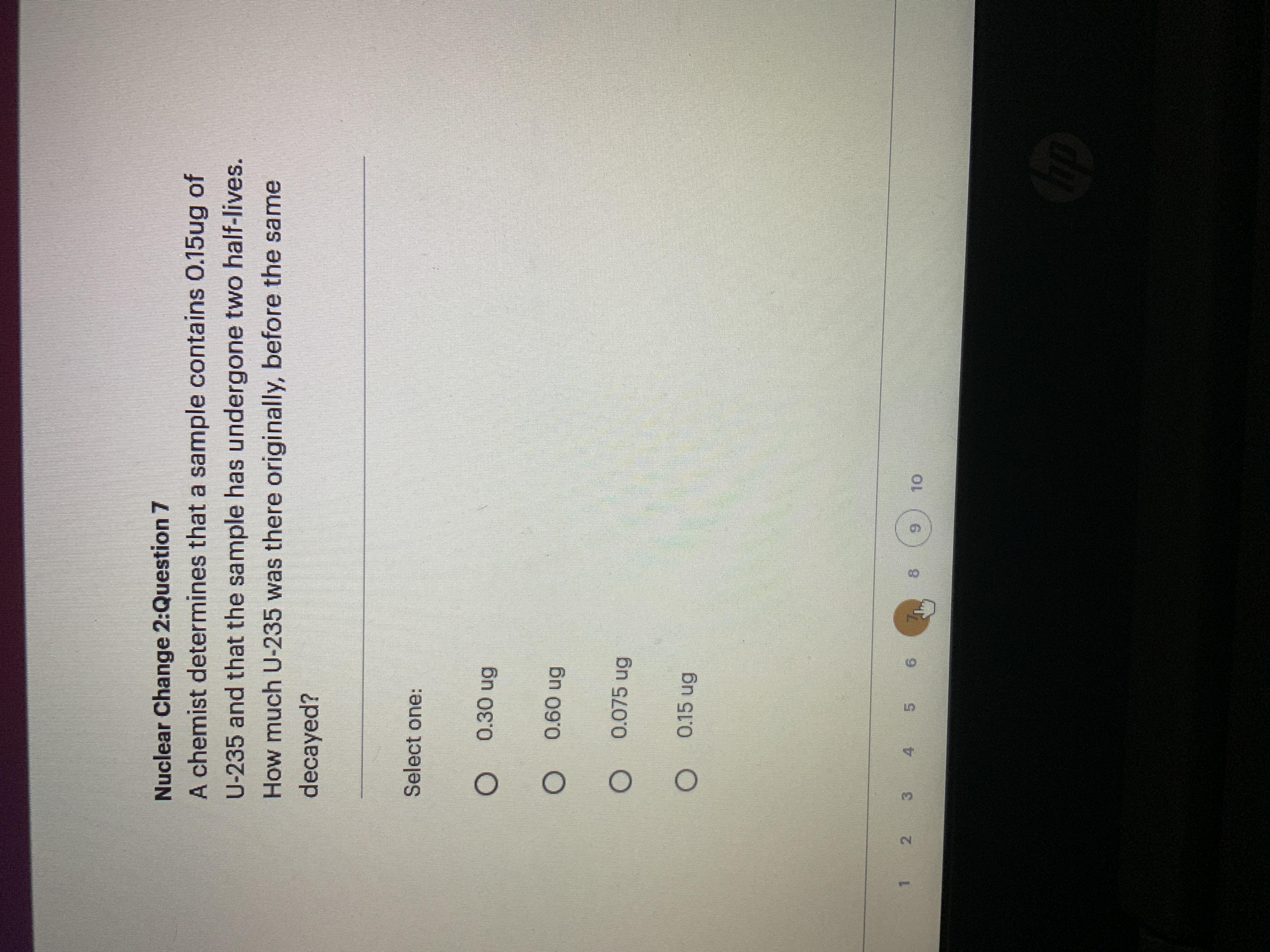
Answers
Answer:
0.30 ug of U-235
Explanation:
N = N0 * (1/2)^(1/2)
0.15 ug = N0 * (1/2)^(1/2)
N0 = 0.15 ug / (1/2)^(1/2)
N0 = 0.30 ug
Answer:
B
Explanation:
The decay of U-235 follows an exponential decay model, which can be described by the equation:
N = N0 * (1/2)^(t/T)
where N is the amount of U-235 at a given time t, N0 is the initial amount of U-235, t is the elapsed time, and T is the half-life of U-235.
In this case, we know that the sample has undergone two half-lives, which means that the elapsed time is:
t = 2 * T
We also know that the amount of U-235 in the sample is 0.15 ug. We can use this information to solve for the initial amount of U-235 (N0):
N = N0 * (1/2)^(t/T)
0.15 = N0 * (1/2)^(2)
0.15 = N0 * (1/4)
N0 = 0.15 / (1/4)
N0 = 0.60 ug
Therefore, the amount of U-235 that was originally present in the sample before it decayed was 0.60 ug. The answer is B) 0.60 ug.
Even more:
It's not A) 0.30 ug because if the sample originally contained 0.30 ug of U-235 and underwent two half-lives, the amount of U-235 remaining would be:N = N0 * (1/2)^(t/T)
N = 0.30 * (1/2)^(2)
N = 0.30 * (1/4)
N = 0.075 ug
This means that the amount of U-235 in the sample after two half-lives would be 0.075 ug, which is not consistent with the given information that the sample contains 0.15 ug of U-235.
Therefore, the correct answer is B) 0.60 ug, which is the initial amount of U-235 that would have been present in the sample before it decayed.
Use this table to answer the questions on Polymer Selection, questions 27 to 31 . What microstructure would you expect to form in polypropylene? Explain your answer.
Answers
the micro structure that would be formed by polypropylene would be a semi-crystalline structure. This is a result of how polymer chains are organized and how the substance behaves during cooling and solidification. Long chains of propylene monomer units make up polypropylene.
These chains are generated during the polymerization process and become intertwined. The molten polypropylene goes through a process known as crystallization as it cools down. The polymer chains arrange themselves into crystalline and amorphous regions in the semi-crystalline
micro structure of polypropylene. In contrast to amorphous sections, which are more randomly structured, crystalline regions are made up of tightly packed, highly ordered polymer chains. The level of crystallinity can change according on the processing circumstances, cooling rate, and molecular weight.
In polypropylene, the creation of the semi-crystalline micro structure gives the substance good mechanical qualities like stiffness, strength, and impact resistance. The amorphous portions offer flexibility and impact resistance, while the crystalline regions contribute to the material's strength.
to know more about polymerization refer to the link below
https://brainly.com/question/1602388
#SPJ4
rock properties such as cleavage, fractures, and bedding planes ______ the mechanical strength of rock and may allow for downhill slippage. multiple choice question. increase stabilize reduce
Answers
Rock properties such as cleavage, fractures, and bedding planes reduce the mechanical strength of rock and may allow for downhill slippage.
The various characteristics of rocks that are of interest and importance to geologists and other professionals who work with rocks and rock materials are known as rock properties. In addition to the classification and physical properties of rocks, the mechanical, hydrologic, thermal, and electrical properties of rocks are also important.
The physical characteristics of rocks include color, texture, and structure. Rock texture, which refers to the size and arrangement of mineral crystals, is used to distinguish rocks. Cleavage, fractures, and bedding planes are all structural features of rocks that have a significant impact on their mechanical strength, and as a result, their stability.
Fractures, cleavage, and bedding planes can significantly lower the strength of rocks. Fractures, which are cracks in the rock's surface, are the most frequent structural feature. Bedding planes, which are layers of rock that have distinct properties, and cleavage planes, which are parallel fractures that give the rock a tendency to break along specific planes, are examples of other structural features. This lack of mechanical strength allows rocks to slide downhill or collapse quickly.
Learn more about Rock properties here: https://brainly.com/question/30173390
#SPJ11
The population of rabbits in a forest is decreasing. foxes in that region are now competing with each other for the limited number of rabbits in that forest. which type of competition exists among these foxes? competition exists among the foxes.
Answers
Rabbits are eaten by predators such as foxes and wild dogs.
What are predators ?In a biological interaction known as predation, one organism—the predator—kills and consumes another—its prey. It belongs to a group of widespread feeding habits that also includes parasitoidism, micropredation (which typically does not result in host death), and parasitism (which always does, eventually). It differs from scavenging on dead prey, even though many predators also scavenge, and it overlaps with herbivory because predatory seed predators and destructive frugivores are also herbivores.
Predators may actively seek for, pursue, or simply wait for their victim while remaining hidden. The predator decides whether to assault its prey after spotting it. This may entail an ambush or a predatory pursuit, sometimes following a prey stalk.
To learn more about predators from the given link:
brainly.com/question/8101519
#SPJ4
Answer: Interspecific competition.
Explanation: Just took this same test.
From the pictures above, how many processes would have NO EFFECT on the temperature of a room?
A)1
B)2
C)3
D)4

Answers
Answer:
b, talking generates no heat and doesn't take away heat
what is weather how to fold clothes
Answers
Answer:
Weather is : the state of the air and atmosphere at a particular time and place : the temperature and other outside conditions (such as rain, cloudiness, etc.) at a particular time and place. And how to fold clothes:
Fold in one side of the shirt towards the centre, about a third.
Fold the sleeve back the other direction, away from the centre.
Long sleeves can be folded again down towards the hem.
Repeat on the other side.
Fold the collar end towards the bottom hem, making a rectangle.
Explanation:
Answer:
Weather is the state of the air and atmosphere at a particular time and place: the temperature and other outside conditions (such as rain, cloudiness, etc.) in a specific time and place. And how to fold clothes
Explanation:
Fold about a third in one side of the shirt towards the center.
Fold the sleeve back in the other direction, away from the center.
Long sleeves can be folded again down towards the hem.
Repeat on the other side.
Fold the collar end towards the bottom hem, making a rectangle.
Which of the following elements is used to make metal products?
oxygen
potassium
calcium
iron
Answers
Its Iron and Potassium because if you look at the periodic table,and you go down the list of metals, you can see what metals are there.
11. An alloy contains 62 % by mass of aluminum and 38% by mass of unknown element .If 10.0
grams of this alloy has a volume 4.20 cm³ ,use the table of density below to identify the
unknown element in the alloy.
Element
Density g/cm³
(A) Beryllium
Copper
8.96
Aluminum
2.70
(B) Copper
Beryllium
1.85
(C) Iron
Iron
7.87
(D) Silver
Silver
10.49
Answers
Based on the given information and the densities provided in the table, the unknown element in the alloy is most likely Beryllium. option(a)
To identify the unknown element in the alloy, we need to compare the density of the alloy with the densities of the elements listed in the table.
The density of the alloy can be calculated using the given information. We know that 10.0 grams of the alloy has a volume of 4.20 cm³. Density is defined as mass divided by volume, so we can calculate the density of the alloy as:
Density = Mass / Volume = 10.0 g / 4.20 cm³ ≈ 2.38 g/cm³
Now, we compare the calculated density of the alloy (2.38 g/cm³) with the densities listed in the table. From the given options, the closest density is that of aluminum, which is 2.70 g/cm³. The alloy's density is lower than the density of aluminum, which means it must contain an element with a lower density than aluminum.
The unknown element in the alloy is most likely Beryllium (option A) with a density of 1.85 g/cm³. The combination of 62% aluminum and 38% beryllium in the alloy would result in a density close to the calculated value of 2.38 g/cm³. option(a)
For such more questions on densities
https://brainly.com/question/1749900
#SPJ8
1)Predict whether the following solutions are acidic, basic or nearly neutral:
(a)
N
a
B
r
(b)
K
2
S
O
3
(c)
N
H
4
N
O
2
(d)
K
2
H
P
O
4
Answers
NaBr: Nearly neutral, K2SO3: Basic, NH4NO2: Acidic, K2HPO4: Basic
NaBr: Sodium bromide is a salt formed from a strong base (NaOH) and a strong acid (HBr). Salts of strong acids and strong bases are neutral, so NaBr is nearly neutral.
K2SO3: Potassium sulfite is a salt formed from a strong base (KOH) and a weak acid (H2SO3). Salts of strong bases and weak acids are basic, so K2SO3 is basic.
NH4NO2: Ammonium nitrite is a salt formed from a weak base (NH3) and a weak acid (HNO2). Salts of weak acids and weak bases can exhibit acidic or basic properties depending on their relative strengths. In this case, ammonium ions (NH4+) act as a weak acid, making NH4NO2 acidic.
K2HPO4: Dipotassium hydrogen phosphate is a salt formed from a strong base (KOH) and a weak acid (H3PO4). Similar to (b), salts of strong bases and weak acids are basic. Therefore, K2HPO4 is basic.
The predicted acidity or basicity of the given solutions is as follows: (a) nearly neutral, (b) basic, (c) acidic, and (d) basic. The nature of the solutions depends on the specific salts formed and the relative strengths of the acids and bases involved.
To learn more about Salts , visit
brainly.com/question/13818836
#SPJ11
What is the pH of 1.0 M KOH solution?
Answers
Answer:
pH = 14
Explanation:
pOH = -log[OH-] = -log1,0 = 0
pH + pOH = 14
pH = 14 - pOH = 14 - 0 = 14
Answer:
pH= 14Explanation:
hope it helps
#CARRYONLEARNING
What type of mixture is a fruit salad
Answers
Answer:
Heterogeneous mixtures
Explanation:
soil rain brand cereal and fruits and are heterogeous
Sodium phosphate reacts with sulfuric acid to form sodium sulfate and phosphoric acid. What is the stoichiometric coefficient for sulfuric acid when the chemical equation is balanced using the lowest whole-number stoichiometric coefficients?.
Answers
When sodium phosphate reacts with sulfuric acid, forming sodium sulfate and phosphoric acid, the stoichiometric coefficient for sulfuric acid in the balanced chemical equation is 3.
In every balanced chemical equation, the total number of individual atoms on the reactant side must be equal to the total number of individual atoms on the product side. The stoichiometric coefficient is the number written in front of each reactants and products that tells how many moles of each are needed in the reaction.
The chemical equation for the given reaction is:
\(Na_{3} PO_{4}\) + \(H_{2} SO_{4}\) = \(Na_{2} SO_{4}\) + \(H_{3} PO_{4}\)
Put the necessary stoichiometric coefficient to balance each element.
Balancing Na:
\(2Na_{3} PO_{4}\) + \(H_{2} SO_{4}\) = \(3Na_{2} SO_{4}\) + \(H_{3} PO_{4}\)
Balancing P:
\(2Na_{3} PO_{4}\) + \(H_{2} SO_{4}\) = \(3Na_{2} SO_{4}\) + \(2H_{3} PO_{4}\)
Balancing S:
\(2Na_{3} PO_{4}\) + \(3H_{2} SO_{4}\) = \(3Na_{2} SO_{4}\) + \(2H_{3} PO_{4}\)
Notice that H and O are already balanced.
Hence, the balanced chemical equation for the reaction is:
\(2Na_{3} PO_{4}\) + \(3H_{2} SO_{4}\) = \(3Na_{2} SO_{4}\) + \(2H_{3} PO_{4}\)
where 3 is the stoichiometric coefficient of sulfuric acid, \(H_{2} SO_{4}\).
Learn more about stoichiometric coefficient here: https://brainly.com/question/6666875
#SPJ4
Carbon buildup can be removed from the metal portion of a pressing comb by immersing the metal portion of the comb in a solution containing _____.
Answers
Carbon buildup can be removed from the metal portion of a pressing comb by immersing it in a solution containing an acid.
When a pressing comb is used for styling hair, it can accumulate carbon buildup over time. This buildup can affect the comb's performance and hinder smooth gliding through the hair.
To remove the carbon buildup, the metal portion of the comb can be immersed in a solution containing an acid. The acid helps to dissolve and break down the carbon deposits, making it easier to clean the comb.
Acids such as vinegar, lemon juice, or citric acid are commonly used for this purpose. These acids have properties that help in dissolving carbon and other residues. The comb should be soaked in the acid solution for a specific period of time, allowing the acid to work on the carbon buildup.
After soaking, the comb can be scrubbed gently with a brush or cloth to remove any remaining residue. Finally, rinsing the comb thoroughly with water and drying it properly completes the process.
Hence, immersing the metal portion of a pressing comb in a solution containing an acid is an effective method to remove carbon buildup and restore the comb's functionality.
Learn more about Carbon here:
https://brainly.com/question/3049557
#SPJ11
Why is the reaction of ethene with bromine called an addition reaction?.
Answers
Answer: an addition reaction is one where two molecules react together to produce one. in this case a bromine molecule reacts with ethene and one of the bromine atoms is added to ethene
Explanation:
hope this helps :)