Answers
Taking into account the definition of calorimetry and latent heat, 11,690 J is required to change 35 g of water from ice to liquid water.
Definition of calorimetryCalorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Definition of sensible heatSensible heat is defined as the amount of heat that a body absorbs or releases without any changes in its physical state (phase change).
Definition of latent heatLatent heat is defined as the energy required by a quantity of substance to change state.
When this change consists of changing from a solid to a liquid phase, it is called heat of fusion and when the change occurs from a liquid to a gaseous state, it is called heat of vaporization.
The heat Q that is necessary to provide for a mass m of a certain substance to change phase is equal to
Q = m×L
where L is called the latent heat of the substance and depends on the type of phase change.
Heat to change water from ice to liquid waterIn this case, you know:
Q= ?m= 35 gL= ΔHfus= 334 \(\frac{J}{g}\) because the change consists of changing from a solid (ice) to a liquid phase (liquid water).Replacing in the definition of latent heat:
Q = 35 g× 334 \(\frac{J}{g}\)
Solving:
Q= 11,690 J
Finally, 11,690 J is required to change 35 g of water from ice to liquid water.
Learn more about calorimetry:
brainly.com/question/14057615
brainly.com/question/24988785
brainly.com/question/21315372
brainly.com/question/13959344
#SPJ1
Related Questions
Given:
180.0 mL chloric acid (HCIO3)
440.0 mL of 1.75 M strontium hydroxide (Sr(OH)2)
Wanted: [HCIO3] necessary to neutralize Sr(OH)?
Answers
The molarity of the 180.0 mL chloric acid, HClO₃ solution needed to neutralize the 440.0 mL of 1.75 M strontium hydroxide, Sr(OH)₂ is 8.56 M
How do i determine the molarity of the chloric acid, HClO₃?We'll begin by writing the balanced equation for the reaction. This is given below:
2HClO₃ + Sr(OH)₂ —> Sr(ClO₃)₂ + 2H₂O
The mole ratio of the acid, HClO₃ (nA) = 2The mole ratio of the base, Sr(OH)₂ (nB) = 1Volume of Sr(OH)₂ (Vb) = 440.0 mLMolarity of Sr(OH)₂ (Mb) = 1.75 M Volume of HClO₃ (Va) = 180.0 mLMolarity of HClO₃ (Ma) =?The molarity of the chloric acid, HClO₃ solution necessary can be obtained as follow:
MaVa / MbVb = nA / nB
(Ma × 180) / (1.75 × 440) = 2
Cross multiply
Ma × 180 = 1.75 × 440 × 2
Divide both side by 180
Ma = (1.75 × 440 × 2) / 180
Ma = 8.56 M
Thus, we can conclude that the molarity of the chloric acid, HClO₃ solution is 8.56 M
Learn more about molarity:
https://brainly.com/question/13386686
#SPJ1
Complete question:
Given that 180.0 mL chloric acid (HCIO3) reacted with 440.0 mL of 1.75 M strontium hydroxide (Sr(OH)2). What is the molarity of HCIO3 necessary to neutralize Sr(OH)?
Wanted: [HCIO3] necessary to neutralize Sr(OH)?
5. The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures
Answers
There are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
To determine the number of water molecules in the given volume of water, we need to use the relationship between mass, volume, and molar mass of water.
First, we need to find the mass of water in the bottle:
Mass = Density * Volume
Mass = 0.967 g/mL * 499.8 mL = 483.9 g
Next, we need to convert the mass of water to moles using the molar mass of water. The molar mass of water (H2O) is approximately 18.015 g/mol.
Moles = Mass / Molar mass
Moles = 483.9 g / 18.015 g/mol = 26.88 mol
Finally, we can calculate the number of water molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules = Moles * Avogadro's number
Number of molecules = 26.88 mol * (6.022 x 10^23 molecules/mol) = 1.62 x 10^25 molecules
Therefore, there are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ8
The pH of a solution after a base and an acid have neutralized each other is ___.
Group of answer choices
A) 6
B) 8
C) 7
D) 0
Answers
Answer: B
Explanation:
Why do electrons repel each other?
Answers
Answer:
Electrons repel each other due to electrostatic force of attraction between both of them as a result prevent the electron from entering the nucleus preventing it from collapsing
Electrons repel each other because they have the same charge present in
them.
What is Law of Magnetism?Law of magnetism states that:
Like poles repelUnlike poles attract.Electrons are subatomic particles which are negatively charged which
depicts them possessing like poles.
This explains why electrons which are in contact with each other will repel as
a result of the repulsive force present in like poles.
Read more about Law of Magnetism here https://brainly.com/question/12529206
The gas phase reaction of H2 with CO2 To produce H2O and CO has…
(Refer to the image, please)

Answers
The given reaction has ΔG value -12207KJ. Therefore, the given reaction is a spontaneous reaction as value of ΔG is negative.
A spontaneous process refers to anything that happens by itself, without external energy input. A ball is going to roll down an incline, water will flow downhill, ice will melt into water, radioactive elements will decay, and iron will rust, for instance. It is impossible for a reaction to not be spontaneous if it is exothermic (H negative) and increases the entropy for the system (S positive). The system's overall heat capacity is measured in enthalpy. The system's unpredictability is gauged by entropy.
ΔG=ΔH-T×ΔS
ΔG=11-298×41
= -12207KJ
Since ΔG is negative, reaction is spontaneous
To know more about spontaneous reaction, here:
https://brainly.com/question/31199175
#SPJ1
After 135 seconds,55g of dubnium-261 has decayed to 1.72g. What is the half life ?
Answers
The half life of the dubnium-261, given that 55 g of it has decayed to 1.72 g after 135 seconds is 27 seconds
How do I determine the half life of the dubnium-261?To obtain the half life of the dubnium-261, we must first determine the number of half lives that has elapsed. This can be obtained as follow:
Original amount of dubnium-261 (N₀) = 55 gAmount remaining of dubnium-261 (N) = 1.72 gNumber of half-lives (n) =?2ⁿ = N₀ / N
2ⁿ = 55 / 1.72
2ⁿ = 32
2ⁿ = 2⁵
n = 5
Now, we shall obtain the half-life of the dubnium-261. Details below:
Time (t) = 135 secondsNumber of half-lives (n) = 5 Half-life (t½) = ?n = t / t½
5 = 135 / t½
Cross multiply
5 × t½ = 135
Divide both sides by 5
t½ = 135 / 5
t½ = 27 secods
Thus, we can conclude that the half-life of the dubnium-261 is 27 seconds
Learn more about half life:
https://brainly.com/question/26374513
#SPJ1
Calculate the pH and percent ionization of a .780 M HNO2 solution.
Answers
Since [H2NO2] is equal to zero in this case, the percent ionization is equal to 100%.
What is ionization ?Ionization is the process of adding an electrical charge to a neutral atom or molecule, resulting in the formation of positively or negatively charged ions. The process involves the transfer of electrons from one atom or molecule to another, which can occur through chemical reactions, physical collisions, or exposure to radiation. Ionization is an important process in many fields, including chemistry, physics, and biology.
The pH of a .780 M HNO2 solution can be calculated using the Henderson-Hasselbalch equation. This equation states that the pH of a solution is equal to the pKa of the acid plus the logarithm of the base to acid ratio. In this case, the pKa of HNO2 is 3.4, so the pH is equal to 3.4 + log(.780/1) = 2.10.
The percent ionization of the solution can be calculated using the formula:
% ionization = ( [HNO2] - [H2NO2] ) / [HNO2] * 100
To learn more about ionization
https://brainly.com/question/30831422
#SPJ1
. Which one is NOT an INDICATOR that a chemical has occurred
A) Gas is produced
B) Precipitate is produced
C) Change in energy
D) Change in mass
Answers
which atom has the largest number of neutrons
Answers
please helpppppppp
MATERIALS
2 shoe boxes or any other boxes that are similar in size
Sheet of clear plastic (plastic wrap) or a pane of glass
2 thermometers for measuring air temperatures
Watch or stop watch
Paper and pen to record temperature results
2 lamps (only needed if it's a cloudy day)
INSTRUCTIONS
Draw a table on your piece of paper to record temperature readings for each box. You can use this template or a table similar to it:
Place a thermometer inside each box; facing up so you can read the temperature.
Tightly cover one of the boxes with clear plastic or glass. Leave the other box open.
Place the boxes in direct sunlight so the bottom of the box is filled with light. If it is a cloudy day, you can use two lamps, setting them directly over each box so that their light shines directly into the boxes. You can use one lamp if it can give equal light to both boxes.
Record the temperature in the boxes once each minute for fifteen minutes.
Remove the boxes from the direct sunlight and continue to record the temperature for another fifteen minutes.
Once you have recorded all of your results, respond to the questions below.
Questions:
In which box did the temperature rise faster?
In which box did the temperature rise higher?
How did the temperatures in the boxes change after removing them from the sunlight?
How does this experiment demonstrate the greenhouse effect?
What does each item used in the experiment represent in the actual greenhouse effect on Earth? For instance, the light used in the experiment represents the Sun. What does the bottom of the box, air in the box, and plastic wrap each represent in the greenhouse effect?
If you can, compare your results with another student and explain the differences or similarities in your findings.
What did you learn from this investigation?


Answers
1. The temperature rose faster in the covered box.
2. The temperature rose higher in the covered box.
3. The temperature in both boxes decreased after removing them from the sunlight.
4. This experiment demonstrates how a greenhouse traps heat inside, causing the temperature to rise.
5. The bottom of the box represents the Earth's surface, the air in the box represents the Earth's atmosphere, and the plastic wrap represents the greenhouse gases that trap heat.
6. You can compare your results with another student to see if your findings are similar or different.
7. From this investigation, you can learn about how greenhouse gases trap heat and cause the Earth's temperature to rise.
What is greenhouse ?
A greenhouse is a structure designed to control the environment for growing plants. It is typically made of glass or other transparent material, and traps heat from the sun, creating a warm and humid environment inside. This helps to extend the growing season and protect plants from extreme weather conditions.
To know more about greenhouse, visit:
https://brainly.com/question/1577730
#SPJ1
write a skeletal equation for the following reaction: An aqueous solution of barium nitrate and an aqueous solution of sodium sulfate are mixed together. Then balance the completed chemical equation.
Answers
Answer:
Ba(NO₃)₂(aq) + Na₂SO₄(aq) ⇒ BaSO₄(s) + 2 NaNO₃(aq)
Explanation:
Let's consider the unbalanced double displacement equation for the reaction of an aqueous solution of barium nitrate and an aqueous solution of sodium sulfate.
Ba(NO₃)₂(aq) + Na₂SO₄(aq) ⇒ BaSO₄(s) + NaNO₃(aq)
To get the balanced equation, we will multiply NaNO₃(aq) by 2.
Ba(NO₃)₂(aq) + Na₂SO₄(aq) ⇒ BaSO₄(s) + 2 NaNO₃(aq)
A cheeseburger from Mchemistry contains 19g of fat, 20g of carbs, and 28g of protein
Answers
Total calories : 363 kcal
Further explanationComplete question
A cheeseburger from a fast-food restaurant contains 19g of fat, 20g of carbs, and 28g of protein. How many kcal of energy does the cheeseburger contain?
We convert each gram of composition to kcal :
1 gram of fat = 9 kcal
1 gram of carbs = 4 kcal
1 gram of proteins = 4 kcal
Fat\(\tt 19\times 9~kcal=171~kcal\)
Carbs\(\tt 20\times 4=80~kcal\)
Protein\(\tt 28\times 4=112~kcal\)
Total calories\(\tt 171+80+112=363~kcal\)
IF I try to dissolve 100 mg of substance X in 100 ml water at 90°C, what
will happen? What kind of solution will result?
Answers
If you try to dissolve 100 mg of substance X in 100 ml water at 90°C, the solubility of substance X will determine the type of solution that will result. Solubility is the maximum amount of a solute that can dissolve in a solvent at a specific temperature.
If substance X is soluble in water at 90°C, it will dissolve in the water to form a homogeneous solution. A homogeneous solution is a mixture of two or more substances that have a uniform composition and appearance. The dissolved substance X will be evenly distributed throughout the water, and the solution will be clear and transparent.
If substance X is not soluble in water at 90°C, it will not dissolve in the water, and a heterogeneous mixture will result. A heterogeneous mixture is a mixture of two or more substances that have a non-uniform composition and appearance. The undissolved substance X will remain as solid particles in the water, and the solution will be cloudy or turbid.
The solubility of substance X in water at 90°C can be influenced by several factors, including temperature, pressure, and the chemical nature of the solute and solvent. If you are unsure about the solubility of substance X in water at 90°C, you can consult reference tables or consult with a qualified chemist for advice.
For more such question substance
https://brainly.com/question/29108029
#SPJ11
I'm having difficult to draw the resonance structure of this molecule. Someone can help me, please

Answers
If 0.499 g of NaOH (MM = 40.00 g/mol) is dissolved in 150.00 mL of water, what is the theoretical molarity of NaOH? (do not forget about SF)
Answers
Molarity is an important method which is used to calculate the concentration of a solution. The molarity of 0.499 g of NaOH dissolved in 150.00 mL of water is 0.082 M.
Molarity of a solution is defined as the number of moles of the solute present per litre of the solution. The unit of molarity is mol L⁻¹ and it is represented as 'M'.
Molarity = Number of moles of solute / Volume of solution in L
150.00 mL = 0.15 L
Number of moles:
n = 0.499 / 40.00 = 0.0124 moles
M = 0.0124 / 0.15
M = 0.082
To know more about molarity, visit;
https://brainly.com/question/16727614
#SPJ1
Name the structure. Group of answer choices 1-chloro-3-cycloheptene 4-chloro-1-cycloheptene 4-chloro-1-cyclohexene 6-chloro-1-cycloheptene
Answers
The correct name of the compound is 4-chloro-1-cycloheptene according to IUPAC nomenclature.
What is IUPAC nomenclature?The IUPAC nomenclature is a system of naming compounds that was put together by the international union of pure and applied chemistry. The system enables the structure of a compound to be easily written from its name.
For the compound shown in the image attached to this answer, the correct name of the compound is 4-chloro-1-cycloheptene according to IUPAC nomenclature.
Learn more about IUPAC nomenclature: https://brainly.com/question/11587934

A warm can of soda has more spray when opened than a cold one. Drag the terms on the left to the appropriate blanks on the right to complete the sentences.
1. more
2. less
3. increased
4. decreased
A. The solubility of a gaseous solute is_____at a higher temperature, and the CO2 pressure in the can is______.
B. When the can of warm soda is opened, ______CO2 is released, producing______spray.
Answers
Answer:
A. The solubility of a gaseous solute is decreases at a higher temperature, and the CO₂ pressure in the can is increased.
B. When the can of warm soda is opened, more CO₂ is released, producing more spray.
Explanation:
We know that , solubility of the gases is inverse proportional to the temperature and directly proportional to the pressure .
We also know that , the kinetic energy of the gases is directly proportional to the temperature of the gas. If the temperature of the gas increase then the kinetic energy of gas will increases that increasing in the kinetic energy of the gas leads to decrease in the solubility of the gases.
When the pressure of the gas increases then the solubility of the gases also will increases because molecule of the gas try to settle in the small area.
From the above we can filled the below blank places
A. The solubility of a gaseous solute is decreases at a higher temperature, and the CO₂ pressure in the can is increased.
B. When the can of warm soda is opened, more CO₂ is released, producing more spray.
Calculate the moles of Iron (III) Sulfide (Fe253) in 218.8 grams. The molar mass of Iron (III) Sulfide is 207.90 g/mol. Do not include units in your response.
Answers
Answer:
1.052
Explanation:
In order to convert from grams of a substance to moles, we need to divide the given mass by the molar mass of the substance:
Moles = Mass / Molar MassAll the required data is given by the problem:
Moles = 218.8 g / 207.90 g/mol = 1.052 molesThere are 1.052 moles in 218.8 grams of iron (III) sulfide.
Help me please omg I don’t know

Answers
Answer:
5 1 2 4and 3 this is correct way
Which statements best support the student’s claim? Select two of the five statements.

Answers
The statements that best support the student’s claim is that
The cells contains rigid structures that supports and protects plant cells.The cells contains structures that converts light energy to chemical energy.What is a cell?A cell is described as the smallest unit that can live on its own and that makes up all living organisms and the tissues of the body.
A cell has three main parts which include :
the cell membrane, the nucleus, and the cytoplasm.The cell membrane surrounds the cell and controls the substances that go into and out of the cell.
In conclusion, Cells are the basic building blocks of all living things.
Learn more about cells at:
https://brainly.com/question/13920046
#SPJ1
help, help,help girlll pleasee help
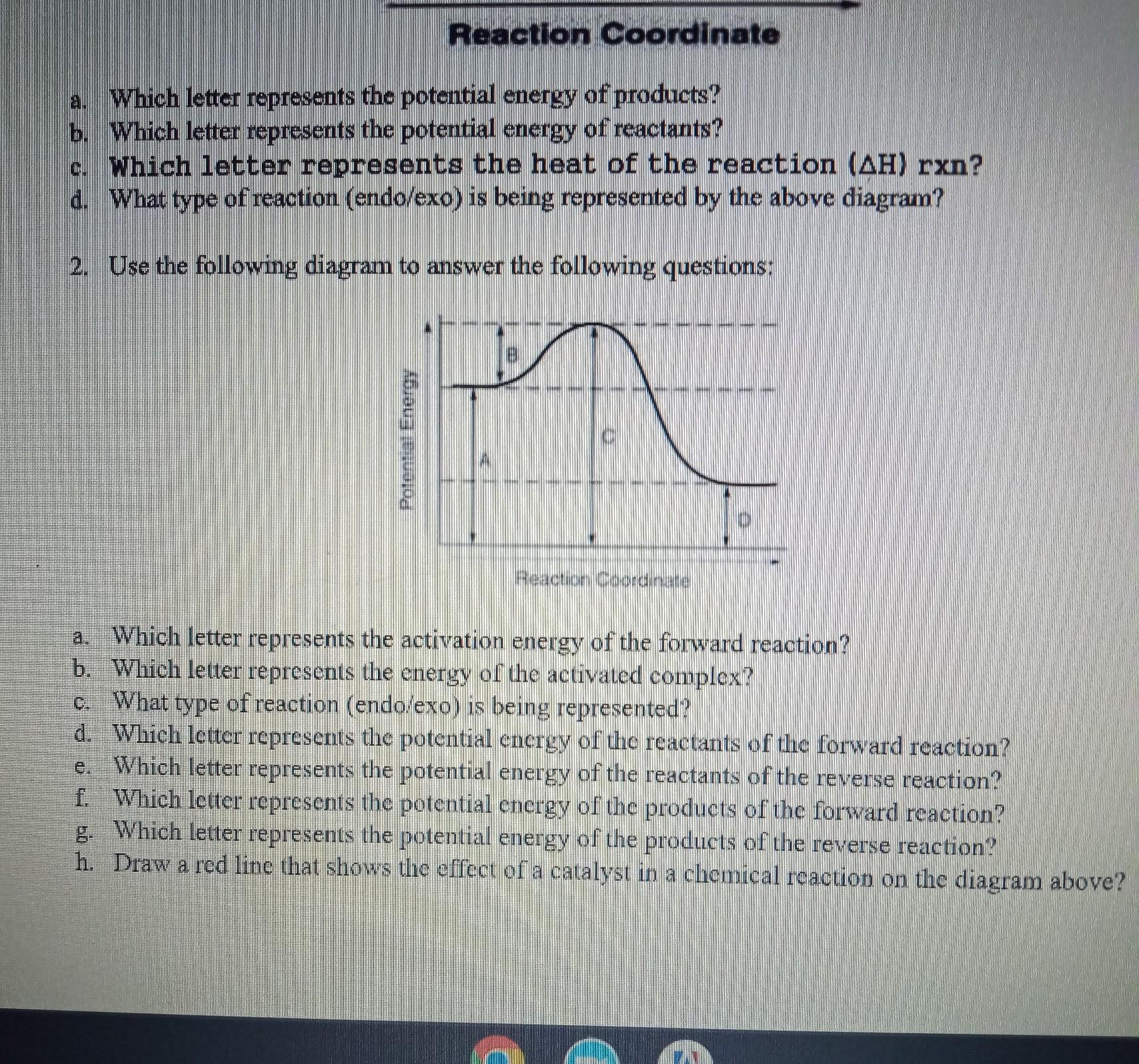
Answers
From the diagram that is shown;
a. The letter B
b. The letter C
c. It is an exothermic reaction
d. Letter A
e. Letter D
f. Letter D
g. Letter A
What is the reaction coordinate?The progression of a chemical reaction from the reactants (beginning materials) to the products (end products) is conceptually represented by the reaction coordinate. It offers a means of observing and evaluating the energy shifts and structural modifications that take place throughout a reaction.
The horizontal axis of a reaction coordinate diagram or energy profile shows how the reaction is progressing, usually from left to right. The system's potential or free energy is shown on the vertical axis. The reaction coordinate can be expressed in terms of separation, bond length, or any other appropriate parameter that accurately characterizes the reaction's progress.
Learn more about reaction coordinate:https://brainly.com/question/30397139
#SPJ1
Given the balanced chemical equation for the decomposition for INO, and the rate of disappearance of INO, write the expressions for the rates of appearance of I2 and NO.
2INO(g) → I2(g) + 2NO(g)
Reactant: Product(I2): Product(NO):
-∆[INO]/2∆t = ??/??
Answers
Answer:
rate of disappearance of -0.5d[INO]/dt
rate of appearance of I2 = d[I2]/dt
rate of appearance of No = 0.5*d[NO]/dt
Explanation:
According to chemical equilibrium, d[I₂]/dt and d[NO]/dt is the expressions for the rates of appearance of I₂ and NO respectively.
What is chemical equilibrium?Chemical equilibrium is defined as the condition which arises during the course of a reversible chemical reaction with no net change in amount of reactants and products.A reversible chemical reaction is the one wherein the products as soon as they are formed react together to produce back the reactants.
At equilibrium, the two opposing reactions which take place take place at equal rates and there is no net change in amount of the substances which are involved in the chemical reaction.At equilibrium, the reaction is considered to be complete . Conditions which are required for equilibrium are given by quantitative formulation.
Factors which affect chemical equilibrium are change in concentration , change in pressure and temperature and presence of catalyst.
Learn more about chemical equilibrium,here:
https://brainly.com/question/4289021
#SPJ6
a gas has an initial volume of 3,480 mL and an initial temperature of - 70.0 C. what must be the temperature of the gas in kelvin if its volume is reduced to 2,450 mL
Answers
The temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
To determine the temperature of the gas in Kelvin after its volume is reduced, we can use the combined gas law, which relates the initial and final conditions of pressure, volume, and temperature for a given amount of gas.
The combined gas law equation is:
(P₁ * V₁) / T₁ = (P₂ * V₂) / T₂
Where P₁ and P₂ are the initial and final pressures, V₁ and V₂ are the initial and final volumes, T₁ is the initial temperature in Kelvin, and T₂ is the final temperature in Kelvin.
Given that the initial volume V₁ is 3,480 mL, the initial temperature T₁ is -70.0 °C (which needs to be converted to Kelvin), and the final volume V₂ is 2,450 mL, we can substitute these values into the equation.
To convert -70.0 °C to Kelvin, we add 273.15 to it, resulting in T₁ = 203.15 K.
Now we can solve for T₂:
(T₂ * V₁) / T₁ = V₂
T₂ = (V₂ * T₁) / V₁ = (2,450 mL * 203.15 K) / 3,480 mL
Simplifying the equation, we find:
T₂ ≈ 143.27 K
Therefore, the temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
A company claims that its weight-loss product is the safest. Why might this
be a biased claim?
A. The company is trying to get you to buy its product.
B. The company has lots of scientific information about the product
C. The company is very successful.
D. The company has studied the product more than anyone.
Answers
Water forms by the reaction 2H2 + O2 ⇄ 2H2O. Suppose the system is allowed to reach equilibrium. What would happen to the concentration of water, if oxygen gas were added to the system? Which reaction would be favored (Forward or Reverse)?
Answers
The concentration of water will increase as the forward reaction is favored.
What is Le Chatelier's principle?According to Le Chatelier's principle, the system will try to counteract the change, either by shifting the equilibrium position to the left (reverse reaction) or to the right (forward reaction). If oxygen gas is added to the system, the equilibrium of the reaction will shift to compensate for the increase in the concentration of O₂.
In this case, the addition of oxygen gas will favor the forward reaction, as the increase in O₂ concentration will drive the equilibrium towards the product side, where H₂O is formed. This is because the forward reaction consumes O₂, and by increasing its concentration, we increase the driving force for the forward reaction.
As a result, the concentration of water will increase as the forward reaction is favored. However, it is important to note that the degree of shift towards the forward reaction will depend on the initial concentrations of H₂, O₂, and H₂O, as well as the temperature and pressure of the system.
Learn more about equilibrium here:
https://brainly.com/question/30807709
#SPJ1
V
A student dissolves 11.S g of sodium hydroxide (NaOH) in 250. g of water in a well-insulated open cup. He then observes the temperature of the water rise
from 20.0 °C to 31.3 °C over the course of 6.7 minutes.
Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction:
NaOH(s) -. Na (ag) + OH (ag)
You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 3
significant digits.
Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and
published values for this reaction.
is this reaction exothermic, endothermic, or neither?
Oexothermic
O endothermic
O neither
0.°
If you said the reaction was exothermic or endothermic, calculate the amount of
heat that was released or absorbed by the reaction in this case.
Calculate the reaction enthalpy AH.
nen per mole of NaOH.
kJ

Answers
According to the question the reaction enthalpy is thus 10610 J / 0.278 moles = 38.3 kJ/mol.
What is enthalpy?Enthalpy is a thermodynamic property of a system that measures the total energy content of a system. It is a state function that is expressed in terms of internal energy, pressure, and volume of a system. Enthalpy represents the amount of energy that is associated with a chemical reaction or physical change.
The reaction is exothermic, meaning that heat is released during the reaction. The amount of heat released can be calculated with the equation q = mcΔT, where q is the heat released, m is the mass of the solution, c is the specific heat capacity of the solution, and ΔT is the change in temperature of the solution. Using the given data, the amount of heat released by the reaction can be calculated as q = (250 g)(4.184 J/g-K)(11.1 K) = 10610 J. The enthalpy change for the reaction can then be calculated by dividing the heat released by the number of moles of NaOH, which is 11.1 g / 40.00 g/mol = 0.278 moles. The reaction enthalpy is thus 10610 J / 0.278 moles = 38.3 kJ/mol.
To learn more about enthalpy
https://brainly.com/question/14047927
#SPJ1
Describe the molecular structure around the indicated atom or atoms:
(a) the sulfur atom in sulfuric acid, (b) the chlorine atom in chloric acid, (c) the oxygen atom in hydrogen peroxide, HOOH
(d) the nitrogen atom in nitric acid, (e) the oxygen atom in the OH group in nitric acid, (f) the central oxygen atom in the ozone molecule, (g) each of the carbon atoms in propyne, (h) the carbon atom in Freon, (i) each of the carbon atoms in allene,
Answers
(a) tetrahedral (b) tetrahedral (c) bent or V-shaped (d) trigonal planar (e) bent or V-shaped (f) bent or V-shaped (g) linear (h) tetrahedral (i) trigonal planar
(a) The molecular structure is tetrahedral around the sulfur atom, with the four oxygen atoms forming the vertices of the tetrahedron.
(b) The molecular structure is tetrahedral around the chlorine atom, with the three oxygen atoms and hydrogen atom.
(c) The molecular structure is bent or V-shaped around the oxygen atom, with the two hydrogen atoms at the bottom of the V.
(d) The molecular structure is trigonal planar around the nitrogen atom, with the three oxygen atoms and hydrogen atom.
(e) The molecular structure is bent or V-shaped around the oxygen atom, with the hydrogen atom and nitrogen atom.
(f) The molecular structure is bent or V-shaped around the central oxygen atom, with the two other oxygen atoms at the bottom of the V.
(g) The molecular structure around each carbon atom is linear, with the two other carbon atoms forming a straight line with the carbon atom at the center.
(h) The molecular structure around the carbon atom is tetrahedral, with the two other carbon atoms and two fluorine atoms forming the vertices of the tetrahedron.
(i) The molecular structure around each carbon atom is trigonal planar, with the two other carbon atoms forming a straight line.
To know more about the molecular structure, here
brainly.com/question/503958
#SPJ4
--The complete question is, Describe the molecular structure around the indicated atom or atoms:
(a) the sulfur atom in sulfuric acid,
(b) the chlorine atom in chloric acid,
(c) the oxygen atom in hydrogen peroxide, HOOH
(d) the nitrogen atom in nitric acid,
(e) the oxygen atom in the OH group in nitric acid,
(f) the central oxygen atom in the ozone molecule,
(g) each of the carbon atoms in propyne,
(h) the carbon atom in Freon,
(i) each of the carbon atoms in allene--
When scientists say that a theory can never be proven, what are they actually saying?
Answers
When scientists say that a theory can never be proven, they mean that it is not possible to conclusively demonstrate its truth. The word "theory" in science refers to a body of knowledge that has been well-established through rigorous testing and observation. However, this does not mean that it is an absolute truth.
It is always subject to revision or even replacement when new evidence emerges or better explanations become available.The scientific method is based on the principle of verifiability, which means that theories must be tested in a way that allows them to be proven false. This is why scientists use experiments, observations, and other methods to test their theories. They look for evidence that supports the theory and also for evidence that contradicts it.If a theory withstands all the tests, it is considered well-supported by the available evidence. However, this does not mean that it is proven beyond a doubt. There is always a chance that new evidence may emerge that contradicts the theory, and this would require revision or replacement of the theory.In summary, scientists say that a theory can never be proven because scientific knowledge is always tentative and subject to revision. Theories can be well-supported by the available evidence, but they can never be proven beyond a doubt.For such more question on contradicts
https://brainly.com/question/30459584
#SPJ8
A student is researching how chemical reactions occur and how temperature impacts the rate of the reaction. She
measures how long it takes for 5 grams of calcite to dissolve in a strong solution of hydrochloric acid at different
temperatures. Her data is shown in the graph

Answers
Based on the data shown in the graph, the rate of reaction is directly proportional to the temperature of a reaction.
What is the rate of a reaction?The speed at which a chemical reaction occurs is called the reaction rate or rate of reaction. The rate of a reaction is proportional to the increase in product concentration per unit time and the decrease in reactant concentration per unit time.
The rate of a reaction is affected by the following:
the temperature of the reaction - the rate of reaction is directly proportional to the temperature of a reaction. Hence, the rate of a reaction increases with an increase in temperature.
presence of a catalyst - the rate of a reaction increases with the addition of a catalyst. A catalyst speeds up the rate of a reaction.
the surface area of the reactants - the rate of a reaction increases with an increase in the surface area of the reactants,
Learn more about the rate of reaction at: https://brainly.com/question/25724512
#SPJ1
Answer:
At higher temperatures, chemical reactions occur more quickly.
Explanation:
edmentum
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100