Answers
Answer:
Explanation: Chemical changes cause a substance to change into an entirely substance with a new chemical formula. And chemical changes are also known as chemical reaction.
Related Questions
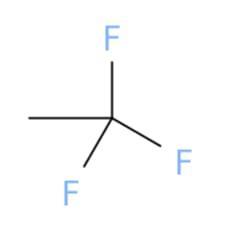
Select the correct structure that
corresponds to the name.
1,1,1-trifluoroethane

Answers
The correct chemical structure that corresponds to 1,1,1-trifluoroethane is (a).
What is 1,1,1-trifluoroethane?
A chemical structure is a spatial arrangement of atoms in a molecule. It determines the molecular geometry and when necessary the electronic chemistry as well .1,1,1-Trifluoroethane or simply known as trifluoroethane is Hydrofluorocarbon (HFC) compound that is colourless and highly inflammable gas with ether like odour. One method of preparation of 1,1,1-Trifluoroethane is by fluorination of 1-chloro-1,1-difluoroethane in the presence of hydrofluoric acid. The chemical formula for 1,1,1-Trifluoroethane is \(C__{2} } H_{3} F_{3}\). The high stability of it's chemical structure because of being heavier than air makes it a greenhouse gas with high infrared absorbent power. It can be used as a propellant or refrigerant and in cleaning of electrical equipments.
Learn more about 1,1,1-trifluoroethane here:
https://brainly.com/question/1390779
#SPJ1
What is the definition of specific heat?
A. The heat needed to raise the temperature of 1 g of a substance
1°C
B. The temperature change between the melting and boiling points of
a substance
C. The heat required to break the molecular bonds within a
substance
OD. The total amount of energy contained within 1 mole of a
substance
SUBMIT
Answers
Answer:
A: The heat needed to raise the temperature of 1 g of a substance1°C
Explanation:
Specific heat is the temperature required to raise the temperature of the unit mass of a given substance. With that being said, of all options A: protrays the definition perfectly.
Side Note: I hope this helps feel free to let me know if you have any other questions.
Give the formula of the conjugate base of HS^-
Answers
The conjugate base of the specie HS^- is S^-
What is the conjugate base?We know that form the Bronsted Lowry perspective of the acid and the base, the acid is the substance that can be able to give out a proton while the base is the kind of substance that can be able to accept a proton.
We can see here that the conjugate base is the specie that can be obtained by the loss of a proton from the HS^- as such we would have it as S^-.
Hence, we can see that the base that is the conjugate base in this sense is S^-.
Learn more about conjugate base:https://brainly.com/question/30225100
#SPJ1
What type of energy does a skier stopped at the top of a hill have because of
his or her position?
A. Kinetic energy
B. Gravitational potential energy
C. Heat energy
D. Chemical energy
Answers
Answer:
B
Explanation:
Given the reaction: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
The reaction occurs more rapidly when a 10-gram sample of Mg is powdered rather than in one piece, because powdered
Mg has
1. less surface area
2. more surface area
3. a lower potential energy
4. a higher potential energy
Answers
Of the bonds below, __________ is the least polar.
A) C,F
B) Na, Cl
C) Si, Cl
D) P,S
E) Na, S
Answers
P-S bond is the least polar.
Polarity is defined as the separation of charge between two atoms participating in a bond, giving rise to a dipole moment and thus a difference in electronegativity(tendency to pull electrons towards itself).
Different atoms have different values of electronegativity
The electronegativity values of the given elements are:
C= 2.55
F=3.98
Na=0.93
Cl=3.16
Si=1.9
P=2.19
S=2.58
Electronegativity difference between:
A) C,F = 3.98-2.55 =1.43
B) Na,Cl = 3.16-0.93=2.23
C) Si,Cl = 3.16-1.9=1.26
D) P,S= 2.58-2.19=0.39
E) Na,S= 2.58-0.93= 1.65
The electronegativity difference between P and S is the least, so P-S bond will be least polar.
To know more about polarity here
https://brainly.com/question/1578097
#SPJ4
Determine the percentage of carbon and hydrogen in ethane C2H6 if the molecular weight is 30.
Answers
Answer:
Percentage of carbon:
\( { \tt{ = \frac{24}{30} \times 100\%}} \\ = 80\%\)
Percentage of hydrogen:
\({ \tt{ = \frac{6}{30} \times 100\%} } \\ = 20\%\)
Calculate the maximum amount of product that can be formed and the amount of unreacted excess reagent when 3.1 mol of SO2 reacts with 2.7 mol of O2 according to the equation: 2SO2(g) + O2(g)->2SO3(g)
I found out that the maximum amount of product that can be produced is 248 g SO3, how can I find the mass of the excess reagent?
Answers
the maximum amount of product that can be formed is 124.39 g SO₃, and there will be 36.8 g of excess O₂ left over.
To find the amount of excess reagent, you need to first determine which reactant is limiting and which is in excess.
Determine the limiting reagent:
Use stoichiometry to determine how much product can be formed from each reactant:
mol SO2:
2 SO₂ + O₂ -> 2 SO₃
2 mol SO₃/2 mol SO₂ = 1 mol SO₃/mol SO₂
1 mol SO₃ = 80.06 g SO₍₃₎
From 2.7 mol O₂
2 SO₂ + O₂ -> 2 SO₃
1 mol SO₃/1 mol O₂ = 1 mol SO₃/mol O₂
1 mol SO₃ = 80.06 g SO₃
2.7 mol O₂ x (1 mol SO₂/1 mol O₂) x (80.06 g SO₂/mol SO₂) = 216.45 g SO₂
Since the amount of SO₂ produced from 3.1 mol of SO₂ is less than the amount produced from 2.7 mol of O₂, SO₂ is the limiting reagent.
Calculate the amount of excess reagent:
To find the amount of excess O₂, use the balanced equation to determine how much O₂ is required to react with all of the SO₂:
2 SO₂ + O₂ -> 2 SO
3.1 mol SO2 x (1 mol O₂/2 mol SO2) = 1.55 mol O₂
Subtract the amount of O₂ used from the initial amount of O₂:
2.7 mol O₂ - 1.55 mol O2 = 1.15 mol O₂
Finally, convert the excess O₂ to mass:
1.15 mol O₂ x 32.00 g/mol = 36.8 g O₂
Learn more about stoichiometry here:
https://brainly.com/question/30215297
#SPJ1
what element is this

Answers
Answer:
Nitrogen (N)
Explanation:
From the question given above, the following data were obtained:
Electronic configuration of the element => 1s² 2s²2p³
Next, we shall determine the number of electrons present in the element.
Thus, the number of electrons in the element can be obtained as follow:
Number of electron = 2 + 2 + 3
Number of electron = 7
Next, we shall determine the number of protons in the element.
Since the element is neutral, the number of protons is the same as the number of electrons. Mathematically,
Number of protons = number of electrons
Number of electron = 7
Therefore,
Number of protons = 7
Finally, we shall determine the atomic number of the element.
The atomic number of an element is the number of protons in the atom of the element. Mathematically,
Atomic number = number of protons
Number of protons = 7
Therefore,
Atomic number = 7
Comparing the atomic number of the element ( i.e 7) with those in the periodic table, the name of the element is Nitrogen (N) since no two elements have the same atomic number.
What do all of the organ systems in a human form?
A,the organism
B.the cells
C.the tissue
D.the organs
Answers
Answer:
A
Explanation:
Cells form tissues
Tissues form organs
Organs for organ systems
Organ systems form organism
Answer:
A the organism
Explanation:
Cause every answer is what is in a human form but the organism is what is made up of all of them
The following Lewis diagram represents the valence electron configuration of a main-group element.
This element is in group
.
According to the octet rule, this element would be expected to form an ion with a charge of
.
If is in period 5, the ion formed has the same electron configuration as the noble gas
.
The symbol for the ion is
.
Answers
This element is in group 1.
According to the octet rule, this element would be expected to form an ion with a charge of +1.
If X is in period 5, the ion formed has the same electron configuration as the noble gas Krypton
The symbol for the ion is Rb⁺
What is electronic configuration?Electronic configuration refers to the arrangement of electrons in the orbitals of an atom or molecule, indicating the energy level of the electrons, the number of electrons in each energy level, and the number of electrons in each orbital.
Considering the given element:
It has one valence electron, hence it is in group 1. Group 1 elements form ions with a charge of +1.
Losing one electron will give the ion the same electron configuration as Kyrton since it is the noble gas in Period 4.
The element is rubidium and the ion is Rb⁺.
Learn more about electronic configuration at: https://brainly.com/question/26084288
#SPJ1

A runner competed in a 5-mile run. How many yards did she run?
a-8800 yards
b-8800 miles
с-8657 yards
Answers
so b. is the correct answer. plz like and hope it helped
What problem occurs if the firework mixture is not pure (i.e., they are dirty or contaminated)?
Answers
Answer:
Fireworks cause broad air contamination in a short measure of time
Explanation:
Fireworks cause broad air contamination in a short measure of time, leaving metal particles, hazardous poisons, destructive synthetic compounds and smoke noticeable all around for quite a long time. A portion of the poisons never completely decay or break down, but instead stick around in the earth, harming all they come into contact with
If the firework mixture is not pure, it is capable of causing risks to health conditions by creating hazardous explosives.
Fireworks originate from the Greek word ''pyrotechnics" which implies fire art. Fireworks is simply a missile that explodes with booms and bursts of vibrantly colorful light in a very controlled fashion.
The chemical components used in the preparation of fireworks are dependent upon the color they produce. These include:
Oxidizing agents such as; barium nitrate (green color), strontium nitrate (red color), etc.fuels such as metal powders, charcoal, etc.If these mixtures are impure, it is certain that these can pose a greater risk related to health conditions by exploding abruptly. These impure gases get mixed with the air and also result in environmental hazards by mixing with the water in lakes, rivers, and streams.
In conclusion, the impurity of firework mixture has a greater effect on human health conditions.
Learn more about fireworks here:
https://brainly.com/question/14533522?referrer=searchResults

What is the percent composition of Fluorine (F) in the compound XeF6?
Od
26.258%
12.520%
110.76%
46.472%
Answers
The percent by mass of the fluorine in the compound is 46.472%.
What is the percent by mass?We know that the percent by mass has to do with the ratio of the total mass of the atom that is part of the compound and the total molar mass of the compound multiplied by one hundred.
The question in this case has demanded that we ought to obtain the mass percent of fluorine from the compound that we can be able to identify from the formula of the compound that is shown here as xenon hexa fluoride.
Mass of the compound can be obtained from; 131 + 6(19)
= 245 g/mol
The total mass of the fluorine atom in the compound is 114 g
Thus we have the use of; 114 /245 * 100/1
= 46.472%
The percent by mass is now gotten for the fluorine atom as 46.472%.
Learn more about percent by mass:https://brainly.com/question/5394922
#SPJ1
describe how sulphate of lead 2 sulphate ammonium chloride can be obtained from a mixture of three
Answers
Steps in separating this mixture include:
Dissolve the mixture in waterAdd a solution of sodium sulfateFilter the precipitate to separate the lead sulfateAdd a solution of ammonium chlorideFilter the precipitate in other to separate lead chloride from the solutionHow to separate sulphate of lead, ammonium chloride, and ammonium sulphate from a mixture of three?A method for extracting sulphate of lead, ammonium chloride and ammonium sulphate out of three different components involves dissolving them first in water before adding sodium sulfate which then leads to formation of solid particles - namely Precipitated Lead Sulfate (PbSO4).
The next step requires removing these particles by employing a filtration process before including Hydrochloric Acid (HCl) so as to derive Ammoniated Lead Chloride (NH4PbCl3).
Again use filtration process on this compound as well followed up with yet another addition step involving Hydrochloric Acid which results in creation of Ammoniated Salt NH4Cl crystals into solution - collect these small gemstones via filtration before undergoing drying procedure thus achieving final products.
Learn about mixture here https://brainly.com/question/2331419
#SPJ1
If 2.45 g of iron are placed in 1,5 L of 0.25M HCl, how many grams of FeCl2 are obtained? Identify the limiting and excess reactants in this single replacement reaction. Fe + 2HCl = FeCl2 + H2
Answers
Answer:
5.56g of FeCl2 can be produced
Explanation:
To solve this question we must find the moles of each reactant. With the moles and the chemical equation we can find limiting reactant. With limiting reactant we can find the moles of FeCl2 and its mass as follows:
Moles HCl:
1.5L * (0.25mol / L) = 0.375 moles HCl
Moles Fe -Molar mass: 55.845g/mol-
2.45g * (1mol / 55.845g) = 0.0439 moles Fe
For a complete reaction of 0.375 moles HCl are needed:
0.375 moles HCl * (1mol Fe / 2mol HCl) = 0.1875 moles Fe
As there are just 0.0439 moles Fe, Fe is limiting reactant
1mol of Fe produce 1 mole of FeCl2, 0.0439 moles Fe produce 0.0439 moles of FeCl2. The mass is:
Mass FeCl2 -Molar mass: 126.751g/mol:
0.0439 moles Fe * (126.751g / mol) =
5.56g of FeCl2 can be producedIn which groups of the modern periodic table are very active metals and very active non-metals placed?
Answers
Answer:
group 1 and are called Alkali metals. Similarly, very active non-metals are placed in group 17
Explanation:
How many kilojoules of heat are needed to raise the temperature of 10g of aluminum from 22 degrees C to 55 degrees C, if the specific heat of aluminum is .901 j/gc?
Answers
Answer:
name four agricultural inputs are subsidized by the government
0.297 kJ of heat is needed to raise the temperature of 10g of aluminum from 22 degrees Celsius to 55 degrees Celsius.
The specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
It is a measure of how much energy it takes to raise the temperature of a substance. It is the amount of heat necessary to raise one mass unit of that substance by one temperature unit.
It is given by the formula -
Q = mcΔT
where, Q = amount of heat
m = mass
c = specific heat
ΔT = Change in temperature
Given,
mass = 10g
c = 0.901J/g⁰C
Initial temperature (T₁) = 22⁰C
Final Temperature (T₂) = 55⁰C
Q = mcΔT
= 10 × 0.901 × (55 -22)
= 297.33 J = 0.297 kJ
Learn more about Specific heat, here:
https://brainly.com/question/31608647
#SPJ1
If the atoms in an ionic bond are not sharing electrons, what keeps the atoms together?
Answers
Answer:
Oppositely charged particles attract each other. This attractive force is often referred to as an electrostatic force. An ionic bond is the electrostatic force that holds ions together in an ionic compound.
Explanation:
Hope this helps :)
How many ions are in 14.02 grams of cyanide
Answers
Answer:
3.25x10²³ ions
Explanation:
To solve this problem we'll first convert those 14.02 grams of cyanide (CN⁻) to moles, using its molar mass:
14.02 g ÷ 26 g/mol = 0.5392 molThen we'll convert those 0.5392 moles into number of ions, using Avogadro's number:
0.5392 mol * 6.023x10²³ ions/mol = 3.25x10²³ ionsWhich of the following is the Arrhenius Theory of acids and bases? O An acid dissociates in water to form Hydrogen ions (H^+) and a base dissociates in water to produce Hydroxide ions (OH^-). O An acid is a proton donor and a base is a proton acceptor. O Acids are substances with a very high pH (greater than 10) and bases are substances with a very low pH (less than 3). O None of the above.
Answers
In water, a highly soluble sodium hydroxide compound dissociates to give sodium ion and hydroxide ion as an example of an Arrhenius base.
To enhance the concentration of hydroxide ions, NaOH entirely dissolves in aqueous solution to give hydroxide ion and sodium ion. The Arrhenius theory, proposed in 1887 by the Swedish scientist Svante Arrhenius, states that acids dissolve in water to produce electrically charged atoms or molecules known as ions, one of which is a hydrogen ion (H+), and that bases ionize in water to produce hydroxide ions (OH). As a result, water is a material that dissociates in water to create H+ ions. It also meets the definition of a material that dissociates in water to create OH ions.
Learn more about hydroxide here-
https://brainly.com/question/4251554
#SPJ4
What happens to the vapor pressure if we mix two volatile liquids (both have measurable vapor pressure)?
PTotal = PA + PB = XA P°A + XB P°B
A.
Vapor pressure is smaller than vapor pressure of either pure liquid.
B.
Vapor pressure is larger than vapor pressure of either pure liquid.
C.
Vapor pressure is between vapor pressures of the pure liquids.
Answers
Answer:
Vapor pressure is between vapor pressures of the pure liquids.
Explanation:
Vapor pressure is between vapor pressures of the pure liquids.
150 mL of 0.25 mol/L magnesium chloride solution and 150 mL of 0.35 mol/L silver nitrate solution are mixed together. After reaction is completed; calculate the concentration of nitrate ions in solution. Assume that the total volume of the solution is 3.0 x 10^2 mL
Answers
Answer:
\(0.175\; \rm mol \cdot L^{-1}\).
Explanation:
Magnesium chloride and silver nitrate reacts at a \(2:1\) ratio:
\(\rm MgCl_2\, (aq) + 2\, AgNO_3\, (aq) \to Mg(NO_3)_2 \, (aq) + 2\, AgCl\, (s)\).
In reality, the nitrate ion from silver nitrate did not take part in this reaction at all. Consider the ionic equation for this very reaction:
\(\begin{aligned}& \rm Mg^{2+} + 2\, Cl^{-} + 2\, Ag^{+} + 2\, {NO_3}^{-} \\&\to \rm Mg^{2+} + 2\, {NO_3}^{-} + 2\, AgCl\, (s)\end{aligned}\).
The precipitate silver chloride \(\rm AgCl\) is insoluble in water and barely ionizes. Hence, \(\rm AgCl\!\) isn't rewritten as ions.
Net ionic equation:
\(\begin{aligned}& \rm Ag^{+} + Cl^{-} \to AgCl\, (s)\end{aligned}\).
Calculate the initial quantity of nitrate ions in the mixture.
\(\begin{aligned}n(\text{initial}) &= c(\text{initial}) \cdot V(\text{initial}) \\ &= 0.25\; \rm mol \cdot L^{-1} \times 0.150\; \rm L \\ &= 0.0375\; \rm mol \end{aligned}\).
Since nitrate ions \(\rm {NO_3}^{-}\) do not take part in any reaction in this mixture, the quantity of this ion would stay the same.
\(n(\text{final}) = n(\text{initial}) = 0.0375\; \rm mol\).
However, the volume of the new solution is twice that of the original nitrate solution. Hence, the concentration of nitrate ions in the new solution would be \((1/2)\) of the concentration in the original solution.
\(\begin{aligned} c(\text{final}) &= \frac{n(\text{final})}{V(\text{final})} \\ &= \frac{0.0375\; \rm mol}{0.300\; \rm L} = 0.175\; \rm mol \cdot L^{-1}\end{aligned}\).
A 13.0kg iron weightlifting plate has a volume of 1650cm^3. What is the density of the iron plate?
Answers
A 13.0kg iron weightlifting plate has a volume of 1650cm³. Therefore, 7.87g/cm³ is the density of iron plate.
What is density?The density of a substance is described as its weight per unit volume. In other words, density is the mass-to-volume ratio or weight per unit volume. It quantifies how much "stuff" a thing possesses inside an unit of volume (cubic meter or cubic centimeter).
Density is a measure of how closely stuff is packed together. Archimedes, a Greek scientist, developed the density principle.
density= mass / volume
= 13000/ 1650
=7.87g/cm³
Therefore, 7.87g/cm³ is the density of iron plate.
To learn more about density, here:
https://brainly.com/question/13434141
#SPJ9
Find the normality of 0.321 g sodium carbonate in a 250 mL solution.
Answers
Answer:
the normality of the given solution is 0.0755 N
Explanation:
The computation of the normality of the given solution is shown below:
Here we have to realize the two sodiums ions per carbonate ion i.e.
N = 0.321g Na_2CO_3 × (1mol ÷ 105.99g)×(2eq ÷ 1mol)
= 0.1886eq ÷ 0.2500L
= 0.0755 N
Hence, the normality of the given solution is 0.0755 N
This is a diagram of fossils found in the same area. Jamie wanted to know which layer of fossils is the oldest, so he
asked his mom. Which layer of fossils did Jamie's mom tell him was the oldest?
A)
Layer 1
B)
Layer 2
C)
Layer 3
D)
Layer 4

Answers
Answer:
D
Explanation:
The bottommost layer is always the oldest.
A radioactive element has a half- life of 2 days. Which fraction represents the amount of an original sample of this element remaining after 6 days?

Answers
Answer : The fraction represents the amount of an original sample of this element remaining after 6 days is \(\frac{1}{8}\) .
Explanation :
Half-life = 2 days
Time = 6 days
Formula used:
\(N=N_o\times (\frac{1}{2})^{(\frac{t}{t_{1/2}})}\)
where,
N = final amount
\(N_o\) = initial amount
t = time
\(t_{1/2}\) = half-life
Now putting all the given values in the above formula, we get:
\(N=N_o\times (\frac{1}{2})^{(\frac{6}{2})}\)
\(\frac{N}{N_o}=(\frac{1}{2})^{(\frac{6}{2})}\)
\(\frac{N}{N_o}=(\frac{1}{2})^3\)
\(\frac{N}{N_o}=\frac{1}{8}\)
Therefore, the fraction represents the amount of an original sample of this element remaining after 6 days is \(\frac{1}{8}\) .
The temperature of 2,000.0 g water is raised 3.00°C. How much
energy is transferred to the water. Use the formula below and use your
notes. The specific heat (Cp) for water is 4.18 J/g • °C. Round to three
significant digits. See your notes and slides.

Answers
Answer:
25 080 j ( Rounded to 25100 j)
Explanation:
FROM THE EQUATION
?? = 4.18 * 2000 * (3) = 25 080 j
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
Determine the mass of 15 mol N.
Answer in units of g.
Answers
Answer:
210.15 g
Explanation:
The molar mass of nitrogen is approximately 14.01 g/mol.
14.01*15=210.15