Answers
Answer:
This is a limitation of the kinetic-molecular theory.
Explanation:
Related Questions
Write a short note on the life of people of ladakh
Answers
Explanation:
They historically leading a migratory shabby chic life with respect to the experiences of humans in Ladakh and are genuine and trustworthy. About the profession of people in Ladakh, 90% of their rely for their subsistence on cultivation dependent on the River Indus. Corn, barley, bulgur, beans, canola and peanuts are their principal staple foods.
O2 is which of the following?
Both compound and molecule
One atom
compound
Molecule
Answers
The chemical formula O₂ which is oxygen is a molecule as it is made up of 2 oxygen atoms.
What is chemical formula?
Chemical formula is a way of representing the number of atoms present in a compound or molecule.It is written with the help of symbols of elements. It also makes use of brackets and subscripts.
Subscripts are used to denote number of atoms of each element and brackets indicate presence of group of atoms. Chemical formula does not contain words. Chemical formula in the simplest form is called empirical formula.
It is not the same as structural formula and does not have any information regarding structure.It does not provide any information regarding structure of molecule as obtained in structural formula.
Learn more about chemical formula,here:
https://brainly.com/question/29031056
#SPJ1
Which of the following is not a characteristic of a compound?
can be decomposed into simpler substances by chemical changes
can be separated by physical means
always in a definite ratio
can obtain new properties by chemical means
Answers
The statement that can be decomposed into simpler substances by chemical changes is not a characteristic of a compound (Option A).
What is a chemical compound?A chemical compound is any substance in nature that cannot be divided into smaller forms and it is part of different molecules by combining its atoms with other atoms of other chemical compounds such as occur with water molecules that form a compound between oxygen and hydrogen.
Therefore, with this data, we can see that a chemical compound is not divided into smaller subunits because it is formed by repeated molecules.
Learn more about chemical compounds here:
https://brainly.com/question/26556885
#SPJ1
Which of the following is a property of acids?
Answers
Answer:
One of the properties of acids is their ability to donate protons (H+ ions) when dissolved in water
Explanation:
One of the properties of acids is their ability to donate protons (H+ ions) when dissolved in water. This property is referred to as acidity. Acids can also be described by the presence of positively charged hydrogen ions (H+) and a pH value below 7. Other properties of acids include their corrosive nature, sour taste, and ability to react with bases to form salts and water in a process called neutralization.
What was the main purpose of the opportunity rover?
Answers
Answer:
Explanation:The major goals of the two rovers, according to NASA, were to determine whether life as we know it could ever have arisen on Mars (focusing particularly on searching for ancient water) and characterizing the climate and geology of
Answer:
The major goals of the two rovers, according to NASA, were to determine whether life as we know it could ever have arisen on Mars (focusing particularly on searching for ancient water) and characterizing the climate and geology of the planet
The smallest possible particle of an element is a(n) .
Answers
Answer: Atoms are the smallest particles of an element.
The amount of 217 mg of an isotope is given by A(t) = 217 € -0.0171, where t is time in years since the initial amount of 217 mg was present. Find the amount to the nearest milligraM left after 20 years
Answers
The amount left after 20 years = 154.15 mg
Further explanationThe atomic nucleus can experience decay into 2 particles or more due to the instability of its atomic nucleus.
Usually radioactive elements have an unstable atomic nucleus.
The main particles are emitted by radioactive elements so that they generally decay are alpha (α), beta (β) and gamma (γ) particles
The decay formula for isotope :
\(\tt \large{\boxed{\bold{A(t)=217e^{-0.0171t}}}\)
Then for t=20 years, the amount left :
\(\tt A(t)=217e^{-0.0171\times 20}\\\\A(t)=154.15~mg\)
Help plz 11 points i need help plz give me TWO not one TWO
!!

Answers
The isotope Ti-48 is produced by the alpha decay of which of the following:
a) ⁵³Mn
b) ⁵⁴Cr
c) ⁵³V
d) ⁵⁴V
e) ⁵²Cr
Answers
Answer:
e) ⁵²Cr
Explanation:
The general form of alpha decay is as follows:
\(\boxed{ ^A_ZX \ \ \rightarrow \ \ ^{A - 4} _{Z - 2} \ Y \ \ + \ \ ^4_2 \alpha}\).
From this, we can see that during alpha decay, the mass number decreases by 4 and the atomic number decreases by 2.
Therefore, we need to find a nucleus that has 4 more nucleons (i.e., a mass number that is 4 more) than that of Ti-48, which is 48 + 4 = 52.
The only option with a nuclear number of 52 is ⁵²Cr, and therefore, Ti-48 is produced by the alpha decay of ⁵²Cr.
A sample of ozone gas occupies 225 mL at 1.00 atm and 0 °C. If the volume of the gas is 625 mL at 25 °C, what is the pressure?
Answers
Answer:
\(P_2=0.393atm\)
Explanation:
Hello,
In this case, we can use the combined ideal gas law in order to analyze its behavior as a function of changing temperature, volume and pressure:
\(\frac{P_1V_1}{T_1} =\frac{P_2V_2}{T_2}\)
Thus, since we know the volume, temperature and pressure at the initial condition, we can compute the final pressure as shown below:
\(P_2=\frac{P_1V_1T_2}{T_1V_2} =\frac{1.00atm*225mL*(25+273)K}{(0+273)K*625mL}\\ \\P_2=0.393atm\)
Regards.
ion
Р
Question 6
1321 ✪
9 words
Consider the reaction 3X + 2Y→ 5C + 4D
How many moles of C can be synthesized from 33.0 moles of Y?
Round your answer to a whole number.
1 pts
Answers
Answer:
83
Explanation:
3X + 2Y → 5C + 4D
2 moles of Y will produce 5 moles of C
33.0 moles of Y will produce: 33.0 x 5/2 = 82.5 or 83 moles of C
Rocks are made of fossils and elements that naturally occur in Earth’s crust.
Answers
Answer:
sedimentary rocks
Explanation:
I did this assignment
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
Assume 0.290 mol N2 and 0.954 mol H2 are present initially.
After complete reaction, how many moles of ammonia are produced?
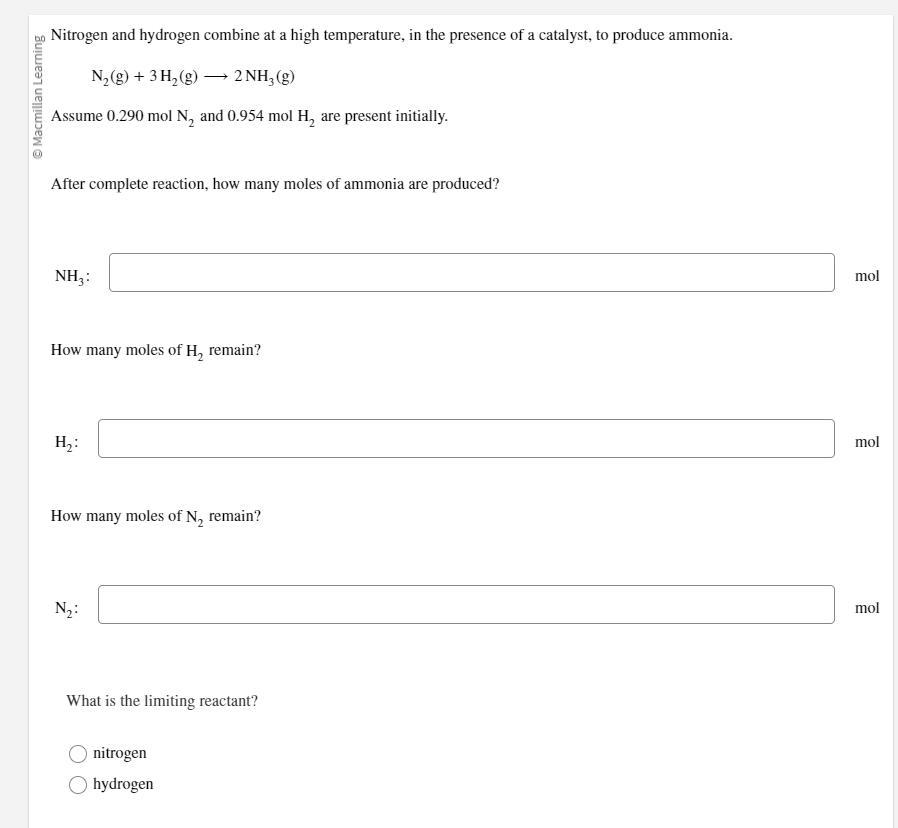
Answers
Answer:
17 reactions
Explanation:
Use the data below to calculate ΔH°rxn for the reaction MgO(s) + CO2(g) à MgCO3(s).
ΔH°f: MgO(s) = -602 kJ mol-1
, CO2(g) = -394 kJ mol-1 and MgCO3(s) = -1096 kJ mol-
Answers
The ΔH°rxn for the reaction MgO(s) + CO2(g) à MgCO3(s) is -100. kJ mol-1.
What is enthalpy ?
Enthalpy is a property or state function that resembles energy; it has the same dimensions as energy and is therefore measured in joules or ergs. The value of enthalpy is solely dependent on the temperature, pressure, and composition of the system, not on its history.
Using ΔH° = ∑ΔfH°(products) – ∑ΔfH°(reactants), the enthalpy change is:
ΔH° = ΔfH°(MgCO3(s)) – [ΔfH°(MgO(s)) + ΔfH°(CO2(g))]
= (-1096 – [-394 -602]) kJ mol-1
= -100. kJ mol-1
To learn more about enthalpy click on the link below:
https://brainly.com/question/27207707
#SPJ9
The contents of two vessels are combined as shown. The balanced chemical equation for the reaction that occurs is also shown. Which of the following represents the constants of the reaction vessel after the N2 and Cl2 react as completely as possible?
Answers
The correct option that represents the constants of the reaction vessel after the N₂ and Cl₂ react as completely as possible is A.
How to determine reaction vessel?The balanced chemical equation for the reaction is:
N₂(g) + 3Cl₂(g) → 2NCl₃(g)
This means that 1 mole of nitrogen gas and 3 moles of chlorine gas react to produce 2 moles of nitrogen trichloride gas.
The contents of the two vessels are:
Vessel 1: 1 mole of N₂
Vessel 2: 3 moles of Cl₂
When these two vessels are combined, the chlorine gas will react completely with the nitrogen gas to produce nitrogen trichloride gas. The remaining gas in the vessel will be nitrogen trichloride gas.
Which is:
[N₂] = 0 mol
[Cl₂] = 0 mol
[NCl₃] = 2 mol
Find out more on reaction vessel here: https://brainly.com/question/30057625
#SPJ1
Complete question:
11 Question 3 A =N O=CI N2 +3 Cl2 2 NC13 The contents of two vessels are combined, as shown. The balanced chemical equation for the reaction that occurs is also shown. Which of the following represents the contents of the reaction vessel after the N2 and Cl2 react as completely as possible?


An element has 2 stable isotopes. One has 13 amu and 1.07% abundant . The second has 12 amu and 98.93% abundant. What is the average atomic mass of the element
Answers
The average atomic mass of the element is 12.0107 amu.
To calculate the average atomic mass of the element in question, we can use the following formula:
average atomic mass = (mass of isotope 1 x abundance of isotope 1) + (mass of isotope 2 x abundance of isotope 2)
where "mass of isotope 1" is the mass of the first stable isotope (13 amu in this case), "abundance of isotope 1" is the percentage of that isotope in the element (1.07% in this case), "mass of isotope 2" is the mass of the second stable isotope (12 amu in this case), and "abundance of isotope 2" is the percentage of that isotope in the element (98.93% in this case).
Substituting the given values in the formula, we get:
average atomic mass = (13 amu x 1.07%) + (12 amu x 98.93%)
average atomic mass = (0.1391 amu) + (11.8716 amu)
average atomic mass = 12.0107 amu
Therefore, the average atomic mass of the element is 12.0107 amu.
This means that on average, one atom of this element weighs 12.0107 atomic mass units (amu), which is slightly heavier than the most abundant isotope (12 amu) due to the presence of the less abundant isotope (13 amu). This concept is important in chemistry because the mass of atoms plays a crucial role in determining their chemical and physical properties. The knowledge of the average atomic mass of an element is important in a wide range of applications, including analytical chemistry, geochemistry, and nuclear physics.
Know more about atomic mass here:
https://brainly.com/question/3187640
#SPJ11
an expression of Avogradros law
Answers
Answer:
The formula for Avagadro's law is V1/n1 = V2/n2, where V = volume and n = amount of gas (in moles).
Explanation:
In the second step of the contact process, sulfur dioxide reacts with to give sulfur trioxide: 2SO₂(g) + O₂(g) = 2SO₂(g) oxygen Initially 10 mol of sulfur dioxide and four moles of oxygen are placed in a container at a specific temperature. The volume of the container is 2 dm³. The equilibrium concentration of oxygen is 0,25 mol.dm. Calculate the equilibrium constant for the reaction.
Answers
The equilibrium constant for the reaction is 0.0052.
How to solveHere are the steps to calculate the equilibrium constant for the reaction:
Write the equilibrium constant expression. The equilibrium constant expression for the reaction is:
K = \([SO3]^2 / [SO2]^2 * [O2]\)
Calculate the equilibrium concentrations of the reactants and products. The equilibrium concentration of oxygen is given as 0.25 mol.dm^3.
One can compute the equilibrium concentration of sulfur dioxide by applying the subsequent formula:
.
\([SO2] = (10 mol - 2 * 0.25 mol) / 2 dm^3 = 4.5 mol.dm^3\)
The equilibrium concentration of sulfur trioxide can be calculated using the following equation:
\([SO3] = (2 * 0.25 mol) / 2 dm^3 = 0.5 mol.dm^3\)
Substitute the equilibrium concentrations into the equilibrium constant expression and solve for K.
K = \((0.5 mol.dm^3)^2 / (4.5 mol.dm^3)^2 * (0.25 mol.dm^3)= 0.0052\)
Therefore, the equilibrium constant for the reaction is 0.0052.
Read more about equilibrium constant here:
https://brainly.com/question/3159758
#SPJ1
Which characteristic is used to classify metamorphic rocks as foliated or non-foliated?
Answers
Answer: d. Arrangement of grains
Explanation:
Edge:)))
When a substance is free from bacteria or microorganisms, that substance is described as being a) clean. b) inert. c) sterile. d) desanitized.
Answers
Answer:
When a substance is free from bacteria or microorganism, that substance is described as being sterile.
Explanation:
Sterile or Sterility:
State of being free from all living microorganisms. In practice, usually described as a probability function, e.g., as the probability of a microorganism surviving sterilization being one in one million.
i need the answer for this

Answers
Answer:
need points
Explanation:
What is the final step of a scientific investigation?
Answers
Answer:
Conclusion
Explanation:
I NEED HELP ON MY SCIENCE ASAP WHAT ARE TWO DIFFERENCES BETWEEN INTRUSIVE AND EXTRUSIVE IGNEOUS ROCKS?
Answers
Answer:
Extrusive and intrusive igneous rocks are the two primary subcategories. Lava, which is magma that has surfaced from beneath the Earth, is what gives rise to extrusive rocks, and they can also be formed by oozing fissures. Meanwhile, Magma cools and solidifies inside the planet's crust, forming intrusive rocks, however because they are inside the earths crust and have solidified there, they are usally the type to penetrate exsisting rocks, inlike extrusive rocks, which form on their own.
Explanation:
I hope this helps, I made this up as I went, with the information i do know. :)
Please help fast
Brianna mixes two liquids together and the solution turns warm. What is an indicator of a chemical
change in the scenario?
A Formation of a gas
B Formation of a precipitate
C Change in color
D Change in energy
Answers
Answer: Change in energy
Explanation: The only evidence provided in the experimental statement is that the "solution turns warm." While the options include positive evidence of a chemical reaction, only "Change in energy" provides both evidence and correlation with an observation.
How many moles are in 4.25 g of C12H22011?
pls help ASAP!
Answers
Answer:
molecular weight of C12H22O11 or grams This compound is also known as Lactose or Sucrose or Maltose. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles C12H22O11, or 342.29648 grams.
Explanation:
342.29648g
How do you find the [H+] concentration?
Answers
2.[H+] = 10−5.6 ≈ 0.0000025 = 2.51 × 10−6 M.
A gas expands and does PV work on its surroundings equal to 322 J. At the same time, it absorbs 132 J of heat from the surroundings. Calculate the change in energy of the gas. Note: PV work means work done by a changing volume against constant pressure. Enter your answer in scientific notation.
Answers
From the calculations, the change in energy is - 190 J.
What is the first law of thermodynamics?From the first law of thermodynamics, the energy is neither created nor destroyed but is transformed from one form to another.
From the law;
U = q + w
U = internal energy
q = heat
w = work
Since work is done on the surroundings and the gas absorbs heat then;
U = 132 J - 322 J
U = - 190 J
Learn more about thermodynamics:https://brainly.com/question/1368306
#SPJ1
PLEASE HELP ME WITH THE QUESTION IN THE IMAGE BELOW I WILL MAKE YOU BRAINLIEST AND GIVE YOU 17 POINTS!

Answers
Answer:
The sound playing from the first graph is louder, while the sound from the second graph is deeper, both of which have the same pitch.
Explanation:
First let's analyze the graphs,
Comparing the first graph to the second
Concluding from the above observations, the sound playing from the first graph is louder, while the sound from the second graph is deeper, both of which have the same pitch.
Which refers to the passing of a wave through an object?
sound
O interference
O transmission
O frequency
O sound
Answers
The term that refers to the passing of a wave through an object is "transmission."
Transmission refers to the process by which a wave passes through an object or medium. In the context of sound, transmission occurs when sound waves travel through different substances, such as air, water, or solids.
When a sound wave encounters an object, it can be transmitted through it, reflected off it, or absorbed by it, depending on the properties of the object and the medium through which the sound is traveling.
For example, when you speak into a microphone, the sound waves produced by your voice travel through the air and are transmitted to the microphone's diaphragm. The diaphragm converts the sound waves into electrical signals, which can then be amplified and reproduced as sound through speakers.
In summary, transmission is the term used to describe the passage of a wave, such as a sound wave, through an object or medium. It is an essential concept in understanding how waves interact with their surroundings and how sound propagates through different materials.
for such more questions on transmission
https://brainly.com/question/18451537
#SPJ8
Please Help ASAP!!
100 pts + Brainliest!!

Answers
Answer:
Hope this helps ;) don't forget to rate this answer !
Explanation:
The molar mass of a sample can be calculated using the formula:
Molar mass = (molecular weight) / (number of moles)
To find the molar mass, we first need to find the molecular weight of the sample. The molecular weight is the weight of a single molecule of the substance in atomic mass units (amu).
To find the molecular weight, we need to convert the weight of the molecule from grams to amu. One amu is equal to 1.66 x 10^-24 grams.
So, to convert the weight of the molecule from grams to amu, we can divide the weight of the molecule (5.34 x 10^-23 grams) by the conversion factor (1.66 x 10^-24 grams/amu):
Molecular weight (amu) = (5.34 x 10^-23 grams) / (1.66 x 10^-24 grams/amu)
= 3.22 x 10^-22 amu
Now that we have the molecular weight, we can use it to calculate the molar mass. However, we need to know the number of moles in order to do this. Without this information, it is not possible to calculate the molar mass.
Answer:
i agree
Explanation: