Calculate the standard enthalpy of reaction for the reaction 2Na(s) +2H2O(l) arrow2NaOH(aq) + H2(g) . Standard enthalpies of formation are -285.8kj/mol for H2O(l) and -470.11kj/mol for NaOH(aq).
Answers
The given reaction has a standard enthalpy of reaction of -368.62 kJ/mol.
Calculation-
The balanced equation for the reaction is:
\(2Na(s) + 2H_2O(l) → 2NaOH(aq) + H_2(g)\)
The following equation can be used to get the reaction's standard enthalpy change (H°rxn):
ΔH° = ΣnΔH°f(products) - ΣnΔH°f(reactants)
the standard enthalpy change of the reaction:
ΔH° = [2(−470.11 kJ/mol) + 0] − [2(0) + 2(−285.8 kJ/mol)]
ΔH° = −940.22 kJ/mol + 571.6 kJ/mol
ΔH°= −368.62 kJ/mol
to know more about enthalpy here;
brainly.com/question/16720480
#SPJ1
Related Questions
Please help !!!!!
Calculate how many joules of energy are needed to warm 4.37 g of silver from 25.0C 27.50 if the specific heat of silver is 0.24 J/g C?
Answers
Answer:
Explanation:
Energy needed = mass * temperature difference * specific heat
= 4.37 * (27.5-25) * 0.24
= 4.37 * 2.5 * 0.24
= 2.622J
Answer:
Explanation:
warm up 2.5C
mass 4.37
specific heat 0.24
energy = 2.5*4.37*0.24
= 2.6J
draw the structural formula for the following compound: 4−isobutyl−1,1−dimethylcyclohexane.
Answers
The structural formula for the following compound is
CH3 CH3
| |
C C
| |
CH2---CH2---CH---CH2---CH3
| |
CH3 CH3
To draw the structural formula for 4-isobutyl-1,1-dimethylcyclohexane, we need to understand the position and arrangement of the different substituents on the cyclohexane ring.
Starting with the cyclohexane ring, it consists of six carbon atoms arranged in a ring structure. We number the carbon atoms from 1 to 6, ensuring that the substituents are given the lowest possible numbers. In this case, we have a methyl group at position 1 and an isobutyl group at position 4.
At position 1 of the cyclohexane ring, we have a methyl group (CH3). This means that there is a single carbon atom attached to the first carbon of the ring, along with three hydrogen atoms.
At position 4 of the cyclohexane ring, we have an isobutyl group. The isobutyl group consists of four carbon atoms, with the central carbon attached to the fourth carbon of the cyclohexane ring. The isobutyl group has the following structure: (CH3)2CHCH2.
Additionally, the name of the compound specifies that there are two dimethyl groups, indicating that two additional methyl groups (CH3) are attached to the cyclohexane ring.
Learn more about cyclohexane at: brainly.com/question/30230108
#SPJ11
Which is a characteristic of the part of the atom marked
"A"?
It contains most of the mass.
It is negatively charged
It is mostly empty space.
It is composed of electrons.
Answers
Answer:
It contains most of the mass
Explanation:
that part A rigth
Answer:
A
Explanation:
12. The smallest unit of an element that has all of the properties of the element is a/an
A. molecule.
B. cell.
C. atom.
D. neutron.
Answers
n atom is a particle of matter that uniquely defines achemical element. An atom consists of a central nucleus that is usually surrounded by one or more electrons. Each electron is negatively charged. The nucleus is positively charged, and contains one or more relatively heavy particles known as protons and neutrons.
Answer:
Explanation:
You have given the exact definition of what an atom is. It is the smallest entity that has all the same properties as a bunch of atoms put together.
What criteria will you use to test whether your hot/cold pack is effective, safe, and easy to use?
Answers
To determine whether a hot or cold pack is effective, safe, and easy to use, several criteria can be considered: effectiveness, safety, and ease of use.
1. Effectiveness: The effectiveness of a hot or cold pack is evaluated based on its ability to relieve pain and reduce inflammation. An effective pack should provide the desired level of relief without causing any adverse effects or complications.
2. Safety: Safety is paramount when using any therapeutic tool. A hot or cold pack should not cause harm to the user. This means it should be designed in a way that prevents burns, bruises, or abrasions to the skin. Proper temperature control is essential to ensure the pack is not too hot or too cold. Sufficient padding should be provided to prevent direct contact with the skin.
3. Ease of use: A hot or cold pack should be user-friendly. It should be easy to handle and should not be overly bulky or heavy. Clear and concise instructions for use should be provided, and the pack should be easy to clean and store. Portability is also important so that the pack can be conveniently carried around.
For a hot or cold pack to be considered effective, safe, and easy to use, it should provide relief from pain and inflammation without causing harm. The pack should have appropriate temperature control and sufficient padding to protect the skin. It should be user-friendly, with clear instructions, easy handling, and convenient portability. Additionally, the pack should be easy to clean and store. By considering these criteria, one can determine whether a hot or cold pack meets the requirements for effective, safe, and easy pain management.
To know more about pack click here:
https://brainly.com/question/1533869
#SPJ11
Two waves travel through each other, and a crest forms with an amplitude smaller that either original waves. What has happened?
Answers
Answer:
This is what I think it is, correct me if I'm wrong. Answer is stated below:
Explanation:
Destructive Interference
Two waves combine to form a wave with a smaller amplitude than either the original wave. Destructive interference can occur when the crest of one wave overlaps the trough of another wave. If the crest has a larger amplitude than the trough of the other wave, a part of it remains.
How do we solve this question? I found B answer key says A

Answers
First, we write the reaction and balance it:
HNO2 (aq) + NH3 (aq) = NH4+ + NO2- (Balanced)
Data:
50 mL of 0.2 M HNO2
50 mL of 0.2 M NH3
In total, we have 100 mL, therefore, this solution between HNO2 and NH3 will be diluted in half. I mean: The concentration of HNO2 and NH3 will be 0.10 M
HNO2 (aq) + NH3 (aq) = NH4+ + NO2-
Initial 0.10 M 0.10 M 0 0
reacts -x -x +x +x
Equilibrium 0.10-x 0.10-x +x +x
Now, we write Kc:
\(\begin{gathered} Kc\text{ = }\frac{\lbrack NH4+\rbrack\lbrack NO2-\rbrack}{\lbrack HNO2\rbrack\lbrack NH3\rbrack}=\frac{x^2}{(0.10-x)^2} \\ 1x10^6=\frac{x^2}{(0.10-x)^2} \\ \sqrt{1x10^6}=\text{ }\lvert{\frac{x}{(0.10-x)}}\rvert \\ We\text{ get 2 values here:} \\ 1)+1000=\frac{x}{(0.10-x)} \\ and \\ 2)-1000\text{ = }\frac{x}{(0.10-x)} \end{gathered}\)Values of x:
For 1) x = 0.0999
For 2)x = 0.1001
We choose number 1) x = 0.0999
Number 2 gives us a value higher than the initial values of concentration
Therefore, concentration in equilibrium of NH3 = 0.10-x =0.10 - 0.0999 = 0.00010M
Answer: A. 0.00010M
if the lab is cold (15° c) the melting point of acetic acid will be __________ that temperature and we will find it as a _________________.
Answers
If the lab is cold at 15°C, the melting point of acetic acid will be lower than that temperature and we will find it as a solid. Acetic acid has a melting point of 16.6°C, which means that it will remain in a solid state at 15°C.
However, if we increase the temperature, the acetic acid will eventually melt and become a liquid. It's important to note that the melting point of a substance can vary depending on the conditions, such as pressure or purity. Therefore, it's essential to control the temperature and other factors when determining the melting point of a substance accurately.
If the lab is cold at 15°C, the melting point of acetic acid, which is around 16.6°C, will be higher than that temperature. As a result, you will find acetic acid in its solid form, known as a crystalline solid, under these conditions.
To know about lab :
https://brainly.com/question/32376341
#SPJ11
!!!PLEASE HELP!!!!
What is the molarity of a solution with 0.5 moles of potassium fluoride dissolved to make 50 mL of solution?
A.0.1 mol/L
B.10 mol/L
C.1.72 mol/L
D.1450 mol/L
Answers
Answer: B
Explanation: molarity = concentration c= n/V = 0.5 mol/ 0.05 l = 10 mol/l
The molarity of a solution is the number of moles of its solute divided by volume in liters. The molarity of the 50 ml solution containing 0.5 moles of potassium fluoride is 10 M or 10 molar.
What is molarity ?Molarity is a common term used to express the concentration of a solution. It is the ratio of number of moles of solute particles to the volume of solution in liters. Hence, its unit is mol/L or molar.
The molarity of a solution is a colligative quantity as well as temperature dependent.
Given,
no.of moles of potassium fluoride salt in water = 0.5 moles.
volume of solution = 50 ml
1 L = 1000 ml
then 50 ml = 0.05 L.
Molarity = no.of moles of solute/ volume of solution in L
M = 0.5 moles/ 0.05 L
= 10 M.
Therefore, the molarity of the given solution is 10 molar.
Find more on molarity:
https://brainly.com/question/8732513
#SPJ6
which reaction will occur? 2NaBr+I2 —> 2NaI + Br2
Answers
Answer:
This is a single replacement reaction because I replaces Br.
Answer: This reaction can'toccur
Explanation
Iodine can't displace Bromine from its compound been less reactive than Br
cartridge brass an fcc substitutional solid solution of zn in cu? is it a two-phase alloy or single-phase alloy?
Answers
Cartridge brass an fcc substitutional solid solution of zinc (Zn) in copper (Cu) is a single-phase alloy.
The FCC stands for face centered cubic lattice. An alloy is defined as a metal that is made up of two or more chemical elements, at least one of which is a metal, and where the atoms are intermixed through metallurgical processing. The atoms of the different elements in an alloy are commonly separated by a few atomic diameters, which distinguishes them from a mixture of metals in which the chemical properties of each component remain unchanged.
There are two types of alloys: single-phase alloys and two-phase alloys. When all of the atoms in an alloy are in the same phase, it is known as a single-phase alloy. In a single-phase alloy, the atoms are uniformly distributed throughout the sample. The material is considered to be homogeneous, or the same throughout, because all of the atoms are in the same phase.
On the other hand, when an alloy contains two different phases, it is known as a two-phase alloy. The constituents are in different phases. In such cases, the phases can either be entirely separated from one another or dispersed. To summarize, cartridge brass is a single-phase alloy because it is an FCC substitutional solid solution of zinc in copper.
Learn more about alloy -
brainly.com/question/1759694
#SPJ11
A current of 3.80 A is passed through a Pb(NO3)2 solution. How long, in hours, would this current have to be applied to plate out 5.70 g of lead
Answers
A current of 3.80 A would need to be applied for 0.24 hours to plate out 5.70 g of lead from a \(Pb(NO_3)_2\) solution.
Q = I × t
moles Pb = (5.70 g)/(331.2 g/mol) = 0.0172 mol
Q = 2 × F × moles Pb
Q = 2 × 96,485 C/mol e- × 0.0172 mol = 3,320 C
t = Q/I = 3,320 C / 3.80 A = 874 seconds
Finally, we convert the time to hours:
t = 874 s / (60 s/min × 60 min/h) = 0.24 hours
A solution typically refers to a homogeneous mixture of two or more substances that are uniformly dispersed throughout each other. The substance that is present in the largest quantity is known as the solvent, while the other substances present in smaller quantities are known as solutes.
Solutions play a crucial role in many areas of physics, including chemistry, material science, and engineering. They can be used to study the properties and behavior of substances, as well as to design and develop new materials with specific properties. The behavior of solutions is governed by several physical laws and principles, including thermodynamics, kinetics, and colloidal chemistry. These laws help us understand phenomena such as osmosis, diffusion, and solubility.
To learn more about Solution visit here:
brainly.com/question/1416865
#SPJ4
Using a periodic table, what is the atomic number of helium?
Answers
Answer:
2
Explanation:
explain how the metal ions produce the color that we see in the flame tests. use the terms 'quantum' and 'energy levels' in your answer.
Answers
The flame test colors of metal ions are based on atomic emission spectra, the atom move from a lower energy level to a higher energy level. that's why metal ions produce the color.
A quantum mechanical system or particle that is bound(that is, confined spatially) can only take on certain discrete values of energy, called energy levels.
When an atom absorbs radiation of a certain wavelength, the electrons contained in the atom move from a lower energy level to a higher energy level. Such a process is called absorption. When this excited electron returns to the ground state, it loses energy, especially color, depending on the frequency of the absorbed radiation. Such a process is called emission. Atoms have different energy levels, so levels closer to the nucleus have less energy than levels further away from the nucleus. That is, an electron moves from a lower energy level to a higher energy level by reaching a certain energy, and after being excited, emits light and returns from a higher energy level to a lower energy level.
learn more about energy level here: https://brainly.com/question/2034726
#SPJ4
If the pH at one-half the first and second equivalence points of a dibasic acid is 4.20 and 7.34, respectively. what are the values for pKa1 and pKa2? From pKa1 and pKa2, calculate the Ka1 and Ka2
Answers
If the pH at one-half the first and second equivalence points of a dibasic acid is 4.20 and 7.34, respectively. The values for pKa1 and pKa2 will be 4.20 and 7.34. Ka1 and Ka2 will be ≈ 6.31 x 10^(-5), and ≈ 2.07 x 10^(-8) respectively.
To find the values of pKa1 and pKa2, we need to understand the relationship between pH and pKa in a titration curve. At one-half the equivalence point, the concentrations of the acid and its conjugate base are equal, resulting in a pH equal to the pKa of the corresponding dissociation.
Given:
pH at one-half the first equivalence point (pKa1) = 4.20
pH at one-half the second equivalence point (pKa2) = 7.34
From the information provided, we can determine the values for pKa1 and pKa2.
pKa1 = pH at one-half the first equivalence point
pKa1 = 4.20
pKa2 = pH at one-half the second equivalence point
pKa2 = 7.34
To calculate Ka1 and Ka2, we use the relationship:
Ka = 10^(-pKa)
Ka1 = 10^(-pKa1)
Ka1 = 10^(-4.20)
Ka2 = 10^(-pKa2)
Ka2 = 10^(-7.34)
Calculating the values:
Ka1 ≈ 6.31 x 10^(-5)
Ka2 ≈ 2.07 x 10^(-8)
To summarize:
pKa1 = 4.20, pKa2 = 7.34
Ka1 ≈ 6.31 x 10^(-5), Ka2 ≈ 2.07 x 10^(-8)
To know more about pKa and titration curves, refer here:
https://brainly.com/question/31502012#
#SPJ11
What must be happening to the forces of attraction between particles when a change of state occurs?
Answers
Answer:
The particles need energy to overcome the attractions between them. As the liquid gets warmer more particles have sufficient energy to escape from the liquid. Eventually even particles in the middle of the liquid form bubbles of gas in the liquid.
HELP ME ASAP PLEASE LOTS OF POINTS
Determine the average atomic mass of Sulfur, given the following:
There are 3 isotopes of the element Sulfur the mass numbers and abundances are as follows:
S-32: 94.99%; S-33: .75%; S-34: 4.26%
Answers
Answer: 32.06 atomic mass units
Explanation:
A compound X is used for disinfecting drinking water supply prepared from slaked lime. (2) a. Identify the compound X b. Write the chemical equation for the preparation of compound X
Answers
Answer:
slaked lime - Ca(OH)2
Ca(OH)2 + CO2 ----> CaCO3(solid) + H2O
X is CO2
Reconstituted ampicillin suspension has a shelf-life for 16 days
when stored in the refrigerator (5°C). What is the shelf-life at
room temperature (25°C)?
Answers
The shelf-life of the reconstituted ampicillin suspension remains unchanged at 16 days when stored at room temperature (25°C) compared to storing it in the refrigerator at 5°C.
To calculate the shelf-life of the reconstituted ampicillin suspension at room temperature, we'll assume that the degradation follows an Arrhenius relationship.
Shelf-life at 5°C (T₁) = 16 days
Temperature at 5°C (T₁) = 5°C
Temperature at room temperature (T₂) = 25°C
To find the shelf-life at room temperature, we can use the Arrhenius equation:
k₁ / k₂ = exp((Ea / R) * (1/T₂ - 1/T₁))
Since we don't have specific values for Ea and the reaction rate constants, we'll assume that they are the same for simplicity. Thus, we can write:
k₁ / k₂ = exp((Ea / R) * (1/25 - 1/5))
Simplifying the equation, we get:
exp((Ea / R) * (4/125)) = 1
To satisfy this equation, the exponential term must be zero, which implies:
(Ea / R) * (4/125) = 0
Solving for Ea, we find:
Ea = 0
Since Ea is zero, it means the reaction rate constants and degradation rates are the same at both temperatures. Therefore, the shelf-life at room temperature (25°C) is the same as the shelf-life at 5°C, which is 16 days.
learn more about shelf-life here:
https://brainly.com/question/27891863
#SPJ4
A cubic centimeter is equal in volume to a _____.
liter
milliliter
deciliter
gram
Answers
Answer:
milliliter
Explanation:
Answer:
B milliliter
Explanation:
Determine whether or not the equation below is balanced. If it isn’t balanced, write the balanced form. Also, identify the reactant(s) and product(s) in this equation. Finally, label this as one of the five types of reactions: combination, decomposition, substitution, double replacement, or reversible.
Answers
Answer:
This is a balanced equation because the same types of atoms and the same numbers of each atom are present on both sides of the equation. 8 S atoms are found on both sides of the equation, and 48 F atoms are found on both sides of the equation. S8 and 24F2 are the reactants; 8SF6 is the product. This is a combination reaction because two substances (S8 and 24F2) undergo a chemical union to form a more complex substance (8SF6).
S8+24F2→8SF6
Explanation:
In a desert ecosystem, coyotes and rattlesnakes both eat the same type of mouse as a primary part of their diets. Recently, coyotes have been hunted and killed by people, decreasing the overall coyote population.
Answers
Answer:
there primary food source will still be the same
Explanation:
they will still ear mice but coyotesmay eat Humans if they are close because once they see humans they think they will be killed its there protectiong
Answer:
D. It will increase because coyotes are the competition.
Explanation:
Because Competition is When 2 organisms fight for the same thing, and sense rattlesnakes are the other competition they will increase because coyotes are being killed.
2. Which statement about activation energy is true?
Answers
Answer: the statement issues are addressed by the people working together is true about activation.
Explanation:
Calculate the mass, in grams, of lithium chloride formed when 15.0g of elemental chlorine completely reacts with lithium.
Answers
Answer:
Explanation:
The relative formular mass for Lithium Chloride is 42.5 This is the sum of the relative atomic masses of Lithium-7 and chlorine-35.5.
Which type of feature forms suddenly where intense compression deforms the rock in an area?
A. A series of rock layers cut by a normal fault
B. A depression that forms a lake
C. A mountain made of volcanic rock
D. A mountain range with folded layers of rock
Answers
D. A mountain range with folded layers of rock.
Intense compression can cause the rock layers to fold, creating a mountain range. This type of feature forms suddenly in the geological timescale, as a result of tectonic activity, and is known as a fold mountain.
The intense pressure causes the rock layers to buckle and deform, resulting in folds, faults, and other features. The Appalachian Mountains and the Rocky Mountains are examples of fold mountains in the United States.
To know more about mountain refer here
https://brainly.com/question/17950058#
#SPJ11
What is the empirical formula of compund that contains 4. 03g of hydrogen per mole, 64. 14 g of sulfur per mole and 128 g of oxyen per mole
Answers
The empirical formula of compound that contains 4. 03g of hydrogen per mole, 64. 14 g of sulfur per mole and 128 g of oxygen per mole is H₂SO₂.
given that :
mass of hydrogen = 4.03 g
mass of sulfur = 64.14 g
mass of oxygen = 128 g
moles of hydrogen = mass / molar mass
= 4.03 / 1
= 4mol
moles of sulfur = 64.14 / 32
= 2 mol
moles of oxygen = 128 / 32
= 4 mol
dividing by the smallest one , we get
mole of H = 2
mole of S = 1
mole of O = 2
The empirical formula = H₂SO₂
Thus, the empirical formula of compound that contains 4. 03g of hydrogen per mole, 64. 14 g of sulfur per mole and 128 g of oxygen per mole is H₂SO₂.
To learn more about empirical formula here
https://brainly.com/question/25780603
#SPJ4
Hey need some help ASAP.
Adult humans have 24 vertebrae in their spinal column. How are these bones classified?
A. long bone
B. irregular bone
C. flat bone
D. short bone
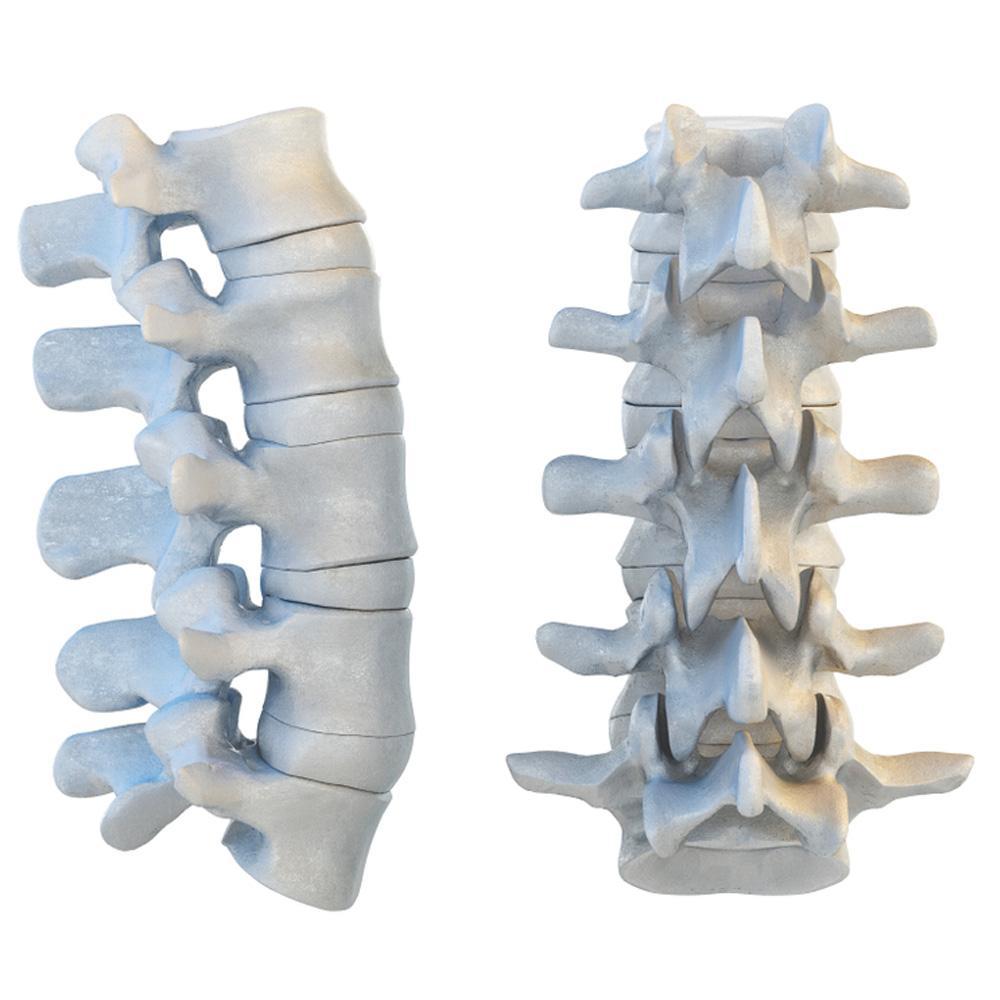
Answers
The vertebrae in the human spinal column are classified as irregular bones. Option B is correct.
Irregular bones have complex shapes that do not fit into other bone classification categories. The vertebrae are irregular because they have a unique structure and shape that allows them to interlock and articulate with each other to form the spinal column.
The spinal column is divided into different regions, including the cervical, thoracic, lumbar, sacral, and coccygeal regions, and each region has a distinct number of vertebrae with specific characteristics. The vertebrae consist of a body, vertebral arch, and various processes for muscle and ligament attachment.
The spinal cord runs through a central canal in the vertebral arch, and nerves branch out between the vertebrae to various parts of the body. Overall, the irregular shape of the vertebrae is critical for providing flexibility, support, and protection to the spinal cord and the body. Option B is correct.
To know more about the Vertebrae, here
https://brainly.com/question/1916134
#SPJ1
Wavelength of yellow light with frequency of 5.2x10 14
Answers
Answer:
5.77x10^-7 m or 577 nm (nanometers)
Explanation:
The wavelength, λ, and frequency, ν, of light are described by the equation:
c = λν
where c is the speed of light.
c = 3.0x10^8 m/s
v = 5.2x10^14
λ = c/v
λ = (3.0x10^8 m/s)/(5.2x10^14) = 5.77x10^-7 m
since 1 m = 10^9nm, we can express this as 577 nm (nanometers)
577 nm. This is in the yellow light span of wavelengths.
Someone pls help me I will make you brain

Answers
Answer:
i guess the answer is..true
the ozone in troposphere protects the earth from ultra violet waves that may cause global warming
12. What is the internationally accepted system for measurement called? Imperial System of Measurement National System of Units O International System of Units (SI) U.S. Customary System of Measurement
Answers
Internationally accepted system for measurement called as international system of units (SI)
The international system of unit (SI) is commonly known as the metric system and is the international standard for measurement and the international treaty of the meter was signed in Paris on may 20 1875 by seventeen countries including the united states and is now celebrated around the globe as world metrology day and long the dominant measurement system used in science
Know more about measurement
https://brainly.com/question/3352797
#SPJ9