Calculate the volume of 0.5 M sodium phosphate needed to react with Cu(NO3)2 (aq) in a Copper Cycle that starts with 0.636 grams of Cu(s). Enter your answer here Upload a photo of your calculations: Please select file(s) Select file(s)
Answers
0.04 liters (or 40 milliliters) of 0.5 M sodium phosphate is required to react with Cu(NO3)₂ in the Copper Cycle, given that we start with 0.636 grams of Cu(s).
The balanced chemical equation for the reaction between Cu and Cu(NO3)₂ is:
Cu(s) + 2NO³⁻(aq) + 2H₃O+(aq) → Cu²⁺(aq) + 2NO₂(g) + 4H₂O(l)
The molar mass of Cu is 63.55 g/mol.
0.636 grams of Cu corresponds to 0.01 moles.
According to the stoichiometry of the balanced equation, 0.01 moles of Cu react with 0.02 moles of Cu(NO3)₂.
To determine the volume of 0.5 M sodium phosphate required for the Copper Cycle reaction with Cu(NO3)₂ (aq):
moles = concentration x volume
volume = moles ÷ concentration
The concentration of sodium phosphate is 0.5 M, and the number of moles of Cu(NO3)₂ required is 0.02 moles. Therefore, the volume of 0.5 M sodium phosphate required is:
volume = 0.02 moles ÷ 0.5 M
volume = 0.04 L
To learn more about copper follow the link:
https://brainly.com/question/13677872
#SPJ4
The complete question is:
Calculate the volume of 0.5 M sodium phosphate needed to react with Cu(NO3)₂ (aq) in a Copper Cycle that starts with 0.636 grams of Cu(s).
Related Questions
PLEASE HELP!!!!!!!!!!!!!!!!!!!

Answers
Answer: nervous tissue to allow heart
epithelial to transmits
connective to forms like
muscle tissue to support bone
Explanation:
king_n1886
What is the energy change per gram of ice when an iceberg composed of pure water, cp = 2.06 j/(gk, is heated from -25°c to -15°c?
Answers
Answer:
\(Q=20.6\frac{J}{g}\)
Explanation:
Hello there!
In this case, according to the equation for the calculation of the heat during a heating process:
\(Q=mC(T_f-T_i)\)
It is possible to compute it per gram of ice by just removing m from the equation by dividing at both sides. Next we plug in the given specific heat and the final and initial temperatures to obtain:
\(Q=2.06\frac{J}{g\°C}[-15\°C-(-25\°C)] \\\\Q=20.6\frac{J}{g}\)
Best regards!
Use the particle theory of matter to explain the changes that a particle of
water would experience as it changes phase from a solid to a liquid and
finally to a gas
Answers
As water changes phase from a solid to a liquid, the particles gain energy, vibrate more vigorously, and break free from their fixed positions. When water changes phase from a liquid to a gas, the particles gain even more energy, move more rapidly, and some of them escape into the air as water vapor.
According to the particle theory of matter, all substances are made up of tiny particles called atoms or molecules. These particles are constantly in motion and have spaces between them. The behavior of these particles explains the changes in phase that water undergoes as it transitions from a solid to a liquid and finally to a gas.
Solid to Liquid (Melting):
When water is in its solid phase, the particles are closely packed and have a fixed arrangement. They vibrate in their positions but cannot move freely. As heat is added to the solid water (ice), the particles gain energy and their vibrations become more vigorous. Eventually, the energy is sufficient to overcome the attractive forces between the particles, causing the solid to melt into a liquid. In the liquid phase, the particles are still close together, but they can now move past each other. The increased energy allows the particles to break free from their fixed positions and move more freely.
Liquid to Gas (Evaporation/Vaporization):
As heat is further added to the liquid water, the particles gain more energy and move even more rapidly. Some of the particles at the surface of the liquid gain enough energy to break away from the attractive forces of neighboring particles and escape into the air. This process is known as evaporation or vaporization. The particles that have evaporated become water vapor or gaseous water molecules. Inside the liquid, the remaining particles continue to move and collide with each other. This constant motion and collision transfer energy throughout the liquid, leading to continuous evaporation until all the liquid is converted to gas.
In summary, as water changes phase from a solid to a liquid, the particles gain energy, vibrate more vigorously, and break free from their fixed positions. When water changes phase from a liquid to a gas, the particles gain even more energy, move more rapidly, and some of them escape into the air as water vapor. The behavior of these particles, their motion, and the energy they possess determine the different physical states of water.
To know more about solid visit:
https://brainly.com/question/24719118
#SPJ11
Calculate 8D of water vapor in isotopic equilib- rium with fresh water whose 8D value is -65%0, assuming that a (liquid-vapor) = 1.090.
Answers
The value of 8D of water vapor in isotopic equilibrium with fresh water whose 8D value is -65%0 is -67.79125‰.
The expression for calculating 8D of water vapor in isotopic equilibrium with fresh water can be given by: 8D = α 8D (vapor) + (1 - α) 8D (liquid). Where,α is a fractionation factor and 8D (vapor) and 8D (liquid) are the deuterium enrichments in water vapor and liquid, respectively.
The value of α is given by:a (liquid-vapor) = 1.090So,α = (a (liquid-vapor) - 1) / (a (liquid-vapor) + 1)α = (1.090 - 1) / (1.090 + 1)α = 0.045So,8D = α 8D (vapor) + (1 - α) 8D (liquid)Given,8D (liquid) = -65‰ (‰ denotes permil, which is equal to parts per thousand)
Substitute the given values in the expression and simplify:8D = 0.045 × 8D (vapor) + (1 - 0.045) × (-65)8D = 0.045 × 8D (vapor) - 61.9258D + 2.79125 = 8D (vapor)
Therefore,8D (vapor) = 8D - 2.79125= -65 - 2.79125= -67.79125‰ (answer)Therefore, the value of 8D of water vapor in isotopic equilibrium with fresh water whose 8D value is -65%0 is -67.79125‰.
To learn more about vapor visit;
https://brainly.com/question/32499566
#SPJ11
an arctic weather balloon is filled with 24.6l of helium gas inside a prep shed. the temperature inside the shed is 7 degrees celsius. the balloon is then taken outside, where the temperature is 7 degrees celsius. calculate the new volume of the balloon. you may assume the pressure on the balloon stays constant at exactly 1 atm. round your answer to 3 significant digits.
Answers
The balloon's new volume is 24.6L. An arctic weather balloon being inflated using 24.6 litres of helium gas in a prep shed. The shed is seven degrees Celsius inside.
When the balloon is hauled outside, it is seven degrees Celsius outside. Any three-dimensional solid's volume is equal to how much room it occupies. One of these solids can be a cube, a cuboid, a cone, a cylinder, or a sphere. Chemical compounds are composed of a large number of comparable molecules (or molecular entities), which are composed of atoms from various elements bonded together by chemical bonds. Because of this, a molecule composed of atoms from a single element is not a compound.
v1/t1 = v2/t2
24.6/7 = v2/7
v2 = 24.6L
v1 = v2
Learn more about helium gas here
https://brainly.com/question/26408362
#SPJ4
How many liters of wine can be held in a wine barrel whose capacity is 30.0 gal? You had been given a new penny to test if it is made up of pure copper or not. You measured the mass of the penny which was 2.49 g. You then find that the penny displaces 0.349 cm3 of water. Is the penny made of pure copper? (Density of pure copper = 8.96 g/cm3)
Answers
The first step in this calculation is to know how many liters is equal to 1 gallon, and the value is 3.785 liters, so now we have to make the following calculation:
1 gal = 3.785 Liters
30.0 gal = x Liters
x = 3.785 * 30.0
x = 114 Liters
What do all atoms of the same elements have in commen
Answers
Answer:
Atoms of the same element always have the same number of protons, same Z, but often have different numbers of neutrons, therefore, different mass numbers.
Every magnet must have what at its ends?
O A. A positive charge and a negative charge
B. A positive charge and a north pole
C. A north pole and a south pole
D. A south pole and another south pole
Answers
Answer:
one end is the north pole and the other is the south pole.
Explanation:
a north pole will attract a south pole; the magnets pull on each other. But the two north poles will push each other away. ... A compass is a tiny magnet balanced on a point so it can turn freely.
What is the acceleration of a 200 gram ice cube if the
net force on the ice cube is 2 N?
Answers
2n = 200 x a then divide by 200 on both sides
A = 0.01 m/s
Answer:
First answer is correct, please let me know if you need any help though!
There is an equal number of protons and __________ in a neutral atom.
A. electrons
B. neutrons
C. protons
D. elements.
Answers
the answer is electrons
Anyone know this pleaase help

Answers
Answer:
Explanation:
I'll answer question 3 for you. But just ask one question at a time.
it's really not fair to ask a bunch of questions.
so for 3 we know it's hydroCarbons. or \(H_{2}\) b/c of how Hydrogen is diatomic, C , for Carbon. Next, we know 8 Carbon atoms have attached themselves. so do you happen to know the Lewis diagram for Hydrogen and Carbon? look it up if not. so we know Carbon is 6 on the periodic chart, so it's normally got 6 electrons. I mentioned Lewis structures b/c now you have to picture how the electrons are going to "stick" the atoms together. Recall they like to be in groups of 8 :P so the diatomic Hydrogen is going to stick to one Carbon atom well. Also, Hydrogen is never the central atom, it just "hangs around" :DD , so I added a picture of the Lewis structure for 8 carbon atoms, but, you can see there are double bond connections and the question you asked doesn't say if those are allowed. I feel like the professor has misguided you in this question. b/c HydroCarbons come in a big variety of complex connections, so almost any answer would be correct. but probably 16 is good. but that's not even an option. Maybe they just want you to recognize that Hydrogen is diatomic and comes in twos. so maybe 10 is the best answer. or A)

refrigerant-134a at 800 kpa and 25°c is throttled to a temperature of -20°c. determine the pressure and the internal energy of the refrigerant at the final state.
Answers
The pressure and the internal energy of the refrigerant at the final state
Known data:80.74kJ/kg.
\($$\begin{aligned}& \mathrm{R}-134 \mathrm{a} \\& P_1=800 \mathrm{kPa} \\& T_1=25^{\circ} \mathrm{C}\end{aligned}$$\)
Unknowns:
\($$\begin{aligned}& T_2=? \\& u_2=?\end{aligned}$$\)
The pressure of the refrigerant in the final state is equal to the saturation pressure at -20 degrees Celsius.
\($$\begin{aligned}& P_2=P_{\text {sat }, \text { at }-20^{\circ} \mathrm{C}}=132.82 \mathrm{kPa} \\& P_2=132.82 \mathrm{kPa}\end{aligned}$$\)
State 1 is compressed liquid. Then the enthalpy is that of the saturated liquid at 25 degrees Celsius.
\(\h_1=h_{f, \text { at } 25^{\circ} \mathrm{C}}=86.405 \mathrm{~kJ} / \mathrm{kg}$$\)
The throttling process of the refrigerant is isentalpic. The state 2 of the refrigerant is in the phase mixing region, so we have to calculate the quality 2.
\(x_2=\left(h_2-h_f\right) /\left(h_g-h_f\right)$$\)
Where:
\($$\begin{aligned}h_2 & =h_1=86.405 \mathrm{~kJ} / \mathrm{kg} \\h_f & =25.49 \mathrm{~kJ} / \mathrm{kg} \\h_g & =238.4 \mathrm{~kJ} / \mathrm{kg}\end{aligned}$$\)\($$\begin{aligned}h_2 & =h_1=86.405 \mathrm{~kJ} / \mathrm{kg} \\h_f & =25.49 \mathrm{~kJ} / \mathrm{kg} \\h_g & =238.4 \mathrm{~kJ} / \mathrm{kg}\end{aligned}$$\)
Substituting:
\(x_2=(86.405-25.49) /(238.4-25.49)=0.2861$$\)
Now let's calculate the final internal energy of the refrigerant, remembering that the temperature is -\($20 \mathrm{C}$\).
\(u_2=u_f+x_2\left(u_g-u_f\right)$$\)
Where :
\($$\begin{aligned}& u_f=25.39 \mathrm{~kJ} / \mathrm{kg} \\& u_g=218.34 \mathrm{~kJ} / \mathrm{kg}\end{aligned}$$\)
Substituting :
\($$u_2=25.39+0.2861(218.84-25.39)=80.74 k J / k g$$\)
\($$u_2=80.74 \mathrm{~kJ} / \mathrm{kg}$$\)
To learn more about internal energy visit:https://brainly.com/question/11278589
#SPJ4
Why is sodium a significant source of calories in both foods
Answers
Answer: Sodium is made up of lots of salt.
Explanation:
Eating a lot of salt can cause your body to retain more water, which can show up on the scale as extra pounds. But we're not just talking about water weight here. High salt diets appear to be linked to higher body fat in particular, the kind of fat that accumulates around your middle.
A sample of iron receives 50 J of heat energy that raises the temperature of the iron to a delta T of 25.0°C. If iron has a specific heat of 0.10 J/g°C, what is the mass of the iron sample?
(Show working out)
Answers
Answer: 20 g
Explanation: heat received Q = m c dT
Q= 50J , dT= 25 C anc c= 0.10 J / g C
And m = Q / c dT
The mass of the iron sample that received the heat is determined as 20 g.
Mass of the iron sampleThe mass of the iron sample is calculated as follows;
Q = mcΔT
where;
m is mass of the iron sampleC is the specific heat capacityΔT is change in temperaturem = Q/cΔT
m = (50) / (0.1 x 25)
m = 20 g
Thus, the mass of the iron sample that received the heat is determined as 20 g.
Learn more about heat here: https://brainly.com/question/13439286
#SPJ2
Suppose your hair grows at the rate 1/32 in. per day. find the rate at which it grows in nanometers per sec- ond. since the distance between atoms in a molecule is:_______
Answers
The rate at which it grows in nanometers per second is 9.19 nm/s.
A filamentous biomaterial, hair is primarily made of proteins, particularly keratin. Dermal follicles produce the protein filament known as hair. Animals can be identified in part by their hair. The human body is covered in follicles that generate thick terminal and fine vellus hair, with the exception of regions of glabrous skin. A healthy head of hair offers some warmth and ultraviolet radiation defence.
The given hair growth rate is 1/32 inch per day.
We must determine the growth rate in nanometers per second.
We know that
1 inch= 2.54x10-⁷ nm
1 day= 86400 s
Using the formula
1/32 inch / day= 1×2.54×10-⁷/32×86400
1/32 inch/day = 9.19 nm/s.
To know about growth rate of hair
https://brainly.com/question/16796356
#SPJ4
is seawater an element, compound, or mixture?
Answers
Can you help plzzz thank you
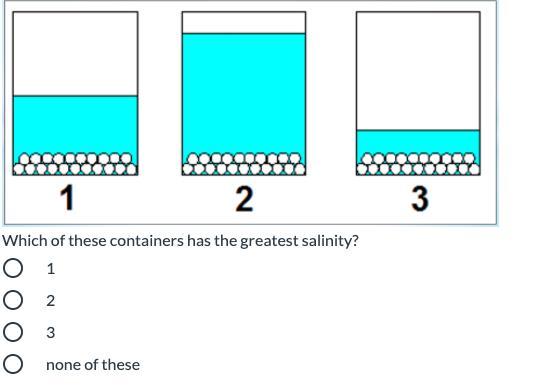
Answers
Answer:
It would be Container 3
Explanation:
Each of the containers has the same amount of salt. Salinity refers to salt level. Since the question is asking for the container with the greatest salinity, you are looking for the container with the least water (because it will be the saltiest out of all of them). Container 3 has the least water.
Hope this helps :)
3 has less water so its saltier
. if your product from this experiment (benzimidazole) is treated with a strong base (nbuli) followed by benzyl bromide (ph-ch2-br) in a polar aprotic solvent like dmso, a molecule c14h12n2 is formed. please draw a reaction scheme of the overall sequence (including the structure of the product), and also an electron-pushing mechanism for each of the reactions that take place.
Answers
However, I can provide a written reaction scheme and mechanism for the reactions involved.
Reaction scheme:
Synthesis of Benzimidazole:
O-phenylenediamine + formic acid → benzimidazole + water
Formation of C14H12N2:
Benzimidazole + nBuLi → intermediate A
Intermediate A + PhCH2Br → C14H12N2 + LiBr
Mechanism:
Synthesis of Benzimidazole:
The reaction between O-phenylenediamine and formic acid is an acid-catalyzed condensation reaction, which proceeds through a series of proton transfers and dehydration steps to yield benzimidazole and water.
Formation of C14H12N2:
The first step involves the deprotonation of the imidazole ring in benzimidazole by nBuLi to generate an intermediate A. The intermediate A is then attacked by the benzyl bromide at the benzylic carbon, which leads to the formation of a new carbon-carbon bond and elimination of the lithium bromide (LiBr) leaving group. The final product is C14H12N2.
To know more about reaction click this link -
brainly.com/question/28984750
#SPJ11
A reaction between 7.88 grams of element X and 23.12 g of elementy produced a compound called XY.
How many grams of XY were produced?
Answers
Answer:
30 g
Explanation:
According to Mendeleev's theory, the total mass of the reactants and products are the same.
Fill in the blanks using terms from this unit.
Even though plants are rooted in the ground, they still move, exert
Blank Options
choose your answer...
and do
Blank Options
choose your answer...
.
Plant cells have very strong cell walls that allow
Blank Options
choose your answer...
to build up inside of the cell as water is absorbed. This pressure is called
Blank Options
choose your answer...
.
When turgor pressure is high enough in a cell, the cell walls become
Blank Options
choose your answer...
and As a result, the cell becomes rigid and the plant is able to stand
Blank Options
choose your answer...
and straight. When a plant does not get enough water, the turgor pressure inside of the cells
Blank Options
choose your answer...
.
A decrease in pushing against the cell wall causes the cells to lose their
Blank Options
choose your answer...
and This causes the plant to begin to droop, or
Blank Options
choose your answer...
.
When the wilted plant gets enough water, the cells will become rigid again, and the plant will stand firm and straight once again.
Answers
Answer:
Even though plants are rooted in the ground, they still move, exert force, and do work.
Plant cells have very strong cell walls that allow pressure to build up inside of the cell as water is absorbed. This pressure is called turgor.
When turgor pressure is high enough in a cell, the cell walls become firm and as a result, the cell becomes rigid and the plant is able to stand tall and straight.
When a plant does not get enough water, the turgor pressure inside of the cells decreases. A decrease in pressure pushing against the cell wall causes the cells to lose their shape and shrink. This causes the plant to begin to droop or wilt.
When the wilted plant gets enough water, the cells will become rigid again, and the plant will stand firm and straight once again.
Explanation:
How do I convert μg/l to mol/kg?
Answers
Answer
To convert μg/l to mol/kg?
Step 1: from the mass in grams, find the number of moles by diving the mass by its molar mass
Step 2: To go from L to kg, take the volume given multiply it by density in kg/L
consider this reaction, which has a kp of 76.7 under certain conditions. co(g) h2o(g) co2(g) h2(g) if the initial pressure of co is 0.415 atm and the initial pressure of h2o is 0.415 atm, what is the equilibrium pressure of h2o (in atm)?
Answers
this reaction, which has a kp of 76.7 under certain conditions. co(g) h2o(g) co2(g) h2(g) if the initial pressure of co is 0.415 atm and the initial pressure of h2o is 0.415 atm, is \(7.34 \text { atm. }\)
What is pressure?
The force delivered perpendicularly to an object's surface per unit area across which that force is dispersed is known as pressure. In comparison to the surrounding pressure, gauge pressure is the pressure. Pressure is expressed using a variety of units.
Let \($\mathrm{x}$\) atm be the equilibrium partial pressure of water vapor.
The equilibrium partial pressure of \($\mathrm{CO}$\) will also be \($\mathrm{x}$\) atm.
The equilibrium partial pressures of \($\mathrm{H}_2$\) and \($\mathrm{CO}_2$\) will be\(10-\mathrm{x} \text\) { atm and } \(20-\mathrm{x}\) atm and \($20-\mathrm{x}\) atm respectively.
\(\text { The equilibrium constant expression is } \mathrm{K}_{\mathrm{p}}\)\(=\frac{\mathrm{P}_{\mathrm{H}_2 \mathrm{O}} \mathrm{P}_{\mathrm{CO}}}{\mathrm{P}_{\mathrm{H}_2} \mathrm{P}_{\mathrm{CO}}}\)
\(\begin{aligned}& 1.60=\frac{\mathrm{x} \times \mathrm{x}}{(10-\mathrm{x}) \times(20-\mathrm{x})} \\& 0.625 \mathrm{x}^2=200-10 \mathrm{x}-20 \mathrm{x}+\mathrm{x}^2 \\& 0.375 \mathrm{x}^2-30 \mathrm{x}+200=0 \\& \mathrm{x}=72.7 \text { or } \mathrm{x}=7.34\end{aligned}\)
The value \($72.7$\) is discarded as the equilibrium partial pressure of hydrogen cannot be negative.
\($$[10-x=10-72.7 < 0]$$\)
\(\text { Hence, the partial pressure of water vapour at equilibrium is } 7.34 \mathrm{~atm} \text {. }\)
To learn more about pressure visit
https://brainly.com/question/12971272
#SPJ4
If a boulder sits at rest on top of a mountain what conclusion can be made about the forces acting on the
Answers
A boulder sits at rest on top of a mountain. What conclusion can be made about the forces acting on the boulder? The forces acting on the boulder are balanced (net force equals zero).
If a boulder sits at rest on the top of the mountain , the forces acting on the boulder are zero as it is in the state of rest.
What is a force?
Force is defined as a cause which is capable of changing the motion of an object. It can cause an object which has mass to change it's velocity. It is also simply a push or a pull . It has both magnitude as well as direction.Hence, it is a vector quantity.
It has SI units of Newton and is represented by'F'.Newton's second law states that force which acts on an object is equal to momentum which changes with time. If mass of object is constant, acceleration is directly proportional to net force acting on an object.
The concepts which related to force are thrust and torque .Thrust increases the velocity of an object and torque produces change in rotational speed of an object.
Learn more about force,here:
https://brainly.com/question/1675020
#SPJ2
Storage areas for dry and canned foods should be kept in a temperature range of between _____.
41 and 50 degrees F
30 and 41 degrees F
50 and 70 degrees F
50 and 70 degrees C
Answers
Answer:
50°F, 70°F
Explanation:
Answer:
50 and 70 degrees F
Explanation:
got it right on the quiz on edge
If a football is filled indoors with 4236 mL of air at a temperature of 25° Celsius, what will happen to the volume of the football if you take it outside on a winter day with an ambient air temperature of 2° Celsius?
It will increase to about ten times the original pressure.
It will decrease by about one tenth of the original pressure.
It will decrease to about half the original pressure
It will increase until it explodes.
Answers
It will decrease by about one tenth of the original pressure..
The volume of gas inside a receptacle affects the pressure inside of it. Using a hand pump to inflate the ball more, the official can correct the problem if the basketball doesn't rebound high enough. He could also let some air out of the ball if it rebounds too high.
Gas pressure-affecting variables
As you may recall from the kinetic-molecular theory, gas particles travel randomly and in straight lines up until an elastic collision occurs with either other gas particles or one of the container walls. The pressure of the gas is determined by these encounters with the container's sides. The state of a gas is described using four factors. They are volume and gravity.
learn more about pressure here:
https://brainly.com/question/29341536
#SPJ1
Please help thank you

Answers
Answer:
The answer is the 2nd one down: The green house affect
A gas has a pressure of 2.36 kPa at 62 °C. What is the pressure at standard
temperature?
Answers
Answer:
P2 = 1.94 kPa
Explanation:
Given the following data;
Initial pressure = 2.36 kPa
Initial temperature = 62°C
Standard temperature = 0°C
Conversion:
Kelvin = 273 + C
Kelvin = 273 + 62 = 335 K
Kelvin = 273 + 0 = 273 K
To find the final pressure, we would use Gay Lussac's law;
Gay Lussac states that when the volume of an ideal gas is kept constant, the pressure of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Gay Lussac's law is given by;
\( PT = K\)
\( \frac{P1}{T1} = \frac{P2}{T2}\)
Making P2 as the subject formula, we have;
\( P_{2}= \frac{P1}{T1} * T_{2}\)
\( P_{2}= \frac{2.36}{335} * 273 \)
\( P_{2}= 0.0071 * 273 \)
P2 = 1.94 kPa
Question
Mutualism, commensalism, and parasitism are all examples of what type of relationship between organisms?
Responses;
harmful
symbiotic
helpful
Answers
Answer:The term "symbiosis" includes a broad range of species interactions but typically refers to three major types: mutualism, commensalism and parasitism. Mutualism is a symbiotic interaction where both or all individuals benefit from the relationship.
Explanation:
geol 101 how is the half-life of a radioactive parent isotope defined? group of answer choices the time it takes for half of the parent isotope to decay half the time it takes for the parent isotope to completely decay the time it takes the parent isotope to go through half the decay steps necessary to produce a stable daughter isotope half of the average rate of decay of the parent isotope
Answers
The half-life of a radioactive parent isotope is defined as the time it takes for half of the parent isotope to decay.
The half-life of a radioactive parent isotope is defined as the time it takes for half of the parent isotope to decay. It is a measure of the stability of a radioactive isotope and is used to predict how long it will take for half of the radioactive atoms in a sample to decay.
This can be used to estimate the age of a sample or to determine how long a specific isotope will remain dangerous. The half-life of a radioactive isotope is a constant and is specific to that isotope. It is not affected by temperature, pressure, or chemical environment. The half-life of a radioactive isotope can range from fractions of a second to billions of years.
To learn more about half-life visit: https://brainly.com/question/12341489
#SPJ4
Your question seems incomplete, but I assume the question was:
"How is the half-life of a radioactive parent isotope defined? (group of answer choices)
The time it takes for half of the parent isotope to decay.
Half the time it takes for the parent isotope to completely decay.
The time it takes the parent isotope to go through half the decay steps necessary to produce a stable daughter isotope.
Half of the average rate of decay of the parent isotope."
Name any two alkaline earth metals