Answers
Answer:
26
Explanation:
Related Questions
A solution contains 5.2 moles of NaCl in 0.5 L of water. What is the molarity of this solution? (Show your work)
Answers
Answer:
10.4 M
Explanation:
5.2 Moles / .5 L = 10.4 M
A balanced chemical equation has equal numbers of atoms of each type on both sides of the equation. This illustrates the principle of
Answers
Answer:
conservation of mass
A sample of oxygen gas at a pressure of 1.19 atm and a temperature of 24.4 °C, occupies a volume of 18.7 liters. If the gas is allowed to expand at constant temperature to a volume of 29.4 liters, the pressure of the gas sample will be ______ atm.
Answers
Answer:
\(\boxed {\boxed {\sf 0.757 \ atm}}\)
Explanation:
We are asked to find the pressure of a gas given a change in volume. Since the temperature remains constant, we are only concerned with volume and pressure. We will use Boyle's Law, which states the volume is inversely proportional to the pressure. The formula for this law is:
\(P_1V_1= P_2V_2\)
Initially, the oxygen gas occupies a volume of 18.7 liters at a pressure of 1.19 atmospheres.
\(1.19 \ atm * 18.7 \ L = P_2V_2\)
The gas expands to a volume of 29.4 liters, but the pressure is unknown.
\(1.19 \ atm * 18.7 \ L = P_2 * 29.4 \ L\)
We are solving for the new pressure, so we must isolate the variable \(P_2\). It is being multiplied by 29.4 liters. The inverse operation of multiplication is division. Divide both sides of the equation by 29.4 L.
\(\frac {1.19 \ atm * 18.7 \ L}{29.4 \ L} =\frac{ P_2 * 29.4 \ L}{29.4 \ L}\)
\(\frac {1.19 \ atm * 18.7 \ L}{29.4 \ L} =P_2\)
The units of liters cancel.
\(\frac {1.19 \ atm * 18.7 }{29.4 } =P_2\)
\(\frac {22.253}{29.4 } \ atm = P_2\)
\(0.7569047619 \ atm =P_2\)
The original measurements all have 3 significant figures, so our answer must have the same. For the number we calculated, that is the thousandth place. The 9 in the ten-thousandth place to the right of this place tells us to round the 6 up to a 7.
\(0.757 \ atm \approx P_2\)
The pressure of the gas sample is approximately 0.757 atmospheres.
According to Boyle's law, for a given mass of ideal gas, pressure of gas is inversely proportional to the volume of gas, Provided the Temprature remains constant.
P₁ = 1.19 atmP₂ = ?V₁ = 18.7 LV₂ = 29.4 LT = constant = 24.4° C = Isothermal process\(\implies \sf P_1 V_1 = P_2 V_2 \\ \)
\(\implies \sf 1.19 \times 18. 7= P_2 \times 29.4 \\\)
\(\implies \sf 22.253= P_2 \times 29.4 \\\)
\(\implies \sf P_2 = \dfrac{22.253}{29.4} \\\)
\(\implies \underline{ \red{\boxed{ \bf P_2 \approx0.756 \: atm }}} \\\)
What is the empirical formula for a
compound that contains 0.126 mol CI
and 0.44 mol O?
Answers
Answer:
In Explanation
Explanation:
The empirical formula of a compound is the simplest whole-number ratio of atoms in a molecule. To find the empirical formula of a compound that contains 0.126 mol CI and 0.44 mol O, we can divide the number of moles of each element by the smallest number of moles among all the elements present. This will give us the simplest ratio of atoms in the compound.
First, we can divide the number of moles of CI by the number of moles of O:
CI/O = 0.126 mol / 0.44 mol = 0.2857
This means that for every atom of oxygen, there are 0.2857 atoms of chlorine.
To simplify this ratio, we can multiply both sides of the equation by the smallest whole number which will make the ratio a whole number. In this case, we can multiply by 4, so the ratio becomes:
CI/O = 4*0.2857 = 1.1428
So the simplified ratio of atoms in the compound is 1 atom of chlorine for every 1 atom of oxygen. The empirical formula is ClO
Answer:
Cl2O7
Explanation:
Didn't round ratio.
The combustion of propane is represented below. For the reaction to occur, the energy of the system must meet the activation energy threshold.
C3H8 (g) + 5 O2 (g) —> 3 CO2 (g) + 4 H2O (g)
Which best explains why increasing the temperature increases the rate of reaction?
A. because the pressure on the system decreases
B. because the reactants become more flammable
C. because carbon dioxide (CO2) traps the additional heat
D. because more molecules collide with greater force and frequency
Answers
Increasing the temperature increases the rate of reaction
D. because more molecules collide with greater force and frequencyEffects of increase of temperatureThe velocity of the particles present in a system simply intensifies with the rise in temperature.
This thermal energy increases their kinetic energy or intense motion, resulting in forceful, frequent collisions between reactant molecules that lead to an upsurge of triumphed ones and subsequently escalate the reaction rate.
Regarding option A, one should note that pressure is unrelated to temperature since they stand as independent variables throughout this reaction. Going for option B, flammability of the reactants isn't susceptible to alterations upon raising the temperature thus making it an unacceptable statement. Lastly, carbon dioxide does not possess heat-trapping properties rather its content solely contributes to diffusing into the overall surroundings.
Learn more about combustion of propane at
https://brainly.com/question/12328568
#SPJ1
Consider the reaction 4FeS2 + 11O2 → 2Fe2O3 + 8SO2. If 8 moles of FeS2 react with 15 moles of O2, what is the limiting reactant? (3 points)
SO2
O2
Fe2O3
FeS2
Answers
Answer:
O2
Explanation:
for find the limiting reactant you must calculate the moles of the reactants from the amount that you have and from the MM:
MM FeS2 = 120n = 26.2g / 120g/mol = 0,218 mol
MM O2 = 32n = 5,44g/32g/mol = 0,17 mol
The limiting reactant is
O2
Si2Br6 compound name
Answers
Answer:
disilicon hexabromide
Explanation:
A spherical balloon of volume 4.06 103 cm3 contains helium at a pressure of 1.25 105 Pa. How many moles of helium are in the balloon if the average kinetic energy of the helium atoms is 3.60 10-22 J
Answers
Convert vol to dm3 by /1000
1.125105*(4.06103/1000)=mol*8.31*temperature
Rearrange for moles
How do you define weight on Earth?
1. the mass of the object plus the mass of Earth
2. the amount of gravitational force between the object and Earth
3. the push or pull that causes the object to move, stop, or change direction
4. the amount of matter in the object sitting on Earth's surface
Answers
Answer:
the amount of gravitational force between the object and Earth
Answer:
2, the gravitational force
When 15.0 mL of a 6.42×10-4 M sodium sulfide solution is combined with 25.0 mL of a 2.39×10-4 M manganese(II) acetate solution does a precipitate form? (yes or no) For these conditions the Reaction Quotient, Q, is equal to .
Answers
Answer:
Q = 3.59x10⁻⁸
Yes, precipitate is formed.
Explanation:
The reaction of Na₂S with Mn(CH₃COO)₂ is:
Na₂S(aq) + Mn(CH₃COO)₂(aq) ⇄ MnS(s) + 2 Na(CH₃COO)(aq).
The solubility product of the precipitate produced, MnS, is:
MnS(s) ⇄ Mn²⁺(aq) + S²⁻(aq)
And Ksp is:
Ksp = 1x10⁻¹¹= [Mn²⁺] [S²⁻]
Molar concentration of both ions is:
[Mn²⁺] = 0.015Lₓ (6.42x10⁻⁴mol / L) / (0.015 + 0.025)L = 2.41x10⁻⁴M
[S²⁻] = 0.025Lₓ (2.39x10⁻⁴mol / L) / (0.015 + 0.025)L = 1.49x10⁻⁴M
Reaction quotient under these concentrations is:
Q = [2.41x10⁻⁴M] [1.49x10⁻⁴M]
Q = 3.59x10⁻⁸
As Q > Ksp, the equilibrium will shift to the left producing MnS(s) the precipitate
Which is considered professional behavior in a laboratory?
trying to be first at everything
showing respect
playing on a cell phone
using a fire blanket to keep warm in class
Answers
Answer:
Showing respect
Explanation:
Being first at everything is immature, playing on a cell phone is being off task, and using a fire blanket to keep warm in class shows that you are doing nothing to contribute to the lab.
I'm so confused, can someone help me?
a) What is the volume of the Ball to the nearest mL?
ball= _____mL
b) This is called the ______method for determining volume.
c) If the mass of the ball is 120. g, calculate the density of the ball and fill in the correct units.
numerical value of the Density:
Units for density. Enter in lower case and as a single line of text.
Also, use standard abbreviations for units. __________
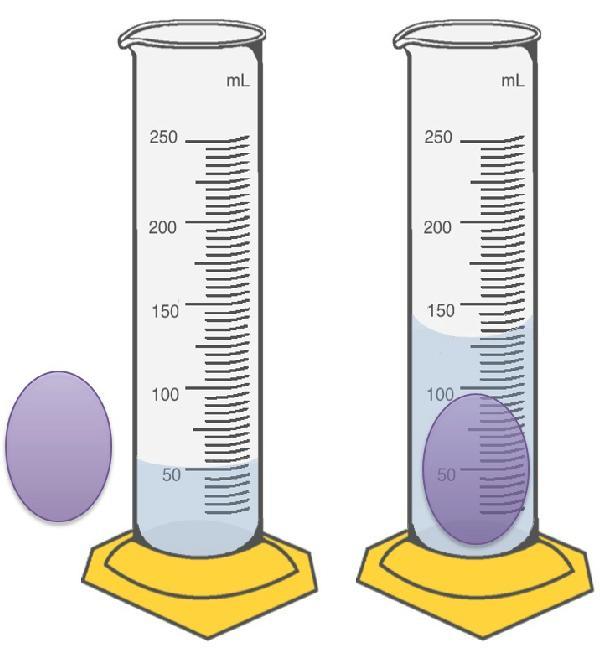
Answers
Answer:
A. 80 mL
B. Water displacement method
C. 1.5 g/mL
Explanation:
A. Determination of the volume of the ball.
Volume of water = 50 mL
Volume of water + ball = 130 mL
Volume of ball =?
Volume of ball = (Volume of water + ball) – (Volume of water)
Volume of ball = 130 – 50
Volume of ball = 80 mL
B. Determination of the name of the method used.
From the above, we can see that the volume of the ball was obtained by calculating the volume of water displaced by the ball.
Thus, the name of the method is WATER DISPLACEMENT METHOD for determining volume of substance.
C. Determination of the density of the ball.
Mass of ball = 120 g
Volume of ball = 80 mL
Density of ball =?
Density = mass / volume
Density of ball = 120 / 80
Density of ball = 1.5 g/mL
A mixture of krypton and argon gas is expanded from a volume of 88.0L to a volume of 100.0L, while the pressure is held constant at 27.0atm. Calculate the work done on the gas mixture. Round your answer to 3 significant digits, and be sure it has the correct sign (positive or negative).
Answers
Answer:
-3.28 × 10⁴ J
Explanation:
Step 1: Given data
Pressure exerted (P): 27.0 atmInitial volume (Vi): 88.0 LFinal volume (Vf): 100.0 LStep 2: Calculate the work (w) done by the gaseous mixture
We will use the following expression.
w = -P × ΔV = -P × (Vf - Vi)
w = -27.0 atm × (100.0 L - 88.0 L)
w = -324 atm.L
Step 3: Convert w to Joule (SI unit)
We will use the conversion factor 1 atm.L = 101.325 J.
-324 atm.L × 101.325 J/1 atm.L = -3.28 × 10⁴ J
Which statement is true concerning the formation of alcohols by the hydroboration-oxidation sequence?
A) overall, the process results in syn addition and Markovnikov orientation
B) overall, the process results in anti addition and Markovnikov orientation
C) overall, the process results in syn addition and anti-Markovnikov orientation
D) overall, the process results in anti addition and anti Markvnikov orientation.
Answers
The statement that is true for the formation of the alcohols by hydroboration-oxidation sequence is C) overall, the process results in syn addition and anti-Markovnikov orientation.
The hydroboration oxidation is the chemical reaction in the alkenes are converted in to the alcohols. this is the two steps process , the step steps includes the hydroboration and the other steps includes the oxidation. The conversion of the alkenes in to the alcohols is done by the anti markovnikov reaction. it is result of the net addition of the water.
Thus , the hydroboration oxidation done by the syn addition and the anti markovnikov orientation.
To learn more about hydroboration oxidation here
https://brainly.com/question/29238473
#SPJ4
How much water has to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M?
Answers
Approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
To find the amount of water that needs to be evaporatedThe relationship between the initial and final concentrations and volumes must be taken into account.
Given: Initial concentration \((C^1) = 1 M Initial volume (V^1) = 250 mL\)
\((C^2) = 3 M final concentration\)
We can use the equation:
\(C^1 * V^1 = C^2 * V^2\)
Where:
\(V^2\)is the final volume of the solution
Rearranging the equation to solve for V2:
\(V^2 = (C^1 * V^1) / C^2\)
Substituting the given values:
\(V^2 = (1 M * 250 mL) / 3 M\)
\(V^2 = 250 mL / 3\)
\(V^2\) ≈ \(83.33 mL\)
To find the amount of water that needs to be evaporated, we subtract the final volume from the initial volume:
Amount of water to be evaporated = \(V^1 - V^2\)
Amount of water to be evaporated = 250 mL - 83.33 mL
Amount of water to be evaporated ≈ 166.67 mL
Therefore, approximately 166.67 mL of water needs to be evaporated from 250 mL of 1 M Ca(OH)2 to make it 3 M.
Learn more about Initial concentration here: brainly.com/question/30720317
#SPJ1
what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.
What does the first law of thermodynamics say?
Answers
According to the first law of thermodynamics, the total energy of a system, including its surrounds, is conserved if heat is acknowledged as a kind of energy.
The study of thermodynamics looks at the connections between heat, work, temperature, and energy. The movement of energy from one place or form to another is the main focus of thermodynamics. The underlying concept is that heat is a form of energy that may be compared to a certain amount of mechanical work.
The heat was not formally recognized as a form of energy until around 1798, when British military engineer Count Rumford (Sir Benjamin Thompson) discovered that endless amounts of heat could be produced while boring cannon barrels and that the amount of heat produced is proportional to the work done while turning a blunt boring tool. Rumford's finding of the relationship between heat produced and work completed served as the theoretical cornerstone for thermodynamics.
To learn more about Thermodynamics please visit-
https://brainly.com/question/1368306
#SPJ9
A gas has a volume of 50.0 mL at a temperature of 10.0 K and a pressure of 760. kPa. What will be the new volume when the temperature is changed to 20.0 K and the pressure is changed to 380. kPa?
Answers
To solve this problem using the gas laws, we need to use the Ideal Gas Law. This law states that the product of the pressure and the volume of a gas is proportional to the absolute temperature.
The equation of the Ideal Gas Law is the following:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{\dfrac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2} } \end{gathered}$} }\)
Where:
P₁ = initial pressure = 760 kPaV₁ = initial volume = 50.0 mL = 0.050 LT₁ = initial temperature = 10.0 KP₂ = Final pressure = 380 kPaT₂ = final temperature = 20.0 KV₂ = Final volume = ?We clear for V₂:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{P_1V_1T_2}{P_2T_1 } } \end{gathered}$} }\)
Where:
P₁ = initial pressure V₁ = initial volumeT₁ = initial temperatureP₂ = Final pressureT₂ = final temperatureV₂ = Final volumeSubstituting the known values:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760\not{kPa}\times0.050 \ L\times20.0\not{k} }{ 380\not{kPa}\times10.0\not{k} } } \end{gathered}$} }\)
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760 \ L}{3800 } } \end{gathered}$} }\)
\(\boxed{\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2\approx0.2 \ Liters} \end{gathered}$} }}\)
When the temperature changes to 20.0 K and the pressure changes to 380 kPa, the new volume will be approximately 0.2 L (200.0 mL).Which of the following characteristics do Element I and Element I have in common?

Answers
Answer:
Option C. The same number of energy levels.
Explanation:
From the diagram given above, element (i) belong to group 2 while element (ii) belong to group 6.
Also, both element i and ii belong to the same period (i.e period 4). This simply means that both element i and ii have the same number of energy levels.
NOTE: Elements in the same period have the same number of shells of electrons which simply means they have the same energy levels.
1. HCI (aq) + NaOH (aq) → NaCl (aq) + H₂O (1)
a. What are the reactants?
b. What are the products?
Answers
In the given reaction, the reactants are hydrochloride acid (HCI) and sodium hydroxide (NaOH). The products are sodium chloride (NaCl) and water.
The chemical equation provided represents a neutralization reaction between hydrochloric acid (HCI) and sodium hydroxide (NaOH) in aqueous solution.
A neutralization reaction is a type of double displacement reaction in which an acid and a base react to form salt and water.
The reactants in this equation are hydrochloric acid (HCI) and sodium hydroxide (NaOH). Hydrochloric acid is a strong acid that dissociates in water to form hydrogen ions (H+) and chloride ions (Cl-). Sodium hydroxide, on the other hand, is a strong base that dissociates in water to form sodium ions (Na+) and hydroxide ions (OH-).
To learn more about reactants, follow the link:
https://brainly.com/question/29816521
#SPJ1
How many carbon dioxide molecules react to form one glucose molecule during photosynthesis
Answers
Answer:
6 molecules
Explanation:
Only three elements are present in the products of photosynthesis: oxygen, carbon, and hydrogen. These same elements are present in the reactants of photosynthesis. Notice that it takes six molecules of water and six molecules of carbon dioxide to make one molecule of glucose
Answer:
6 molecules of carbone dioxide.
ONLY ANSWER IF YOU PLAY ADOPT ME ON ROB.LOX!!! tell me your best pet and user name in your answer plz.
READ!!!!! p.s. i have a fly ride T rex and more! also, STAY ON THE PAGE SO I COULD RESPOND AND WE COULD PLAY! yay!! :) :)
Answers
Answer:
I play!!!!!!!!
Explanation:
Be sure to answer all parts. A student is given four solid samples labeled W, X, Y, and Z. All have a metallic luster. She is told that the solids could be gold, lead sulfide (PbS), quartz which is SiO2, and iodine. The results of her investigations are: (a) W is a good electrical conductor; X, Y, and Z are poor electrical conductors. (b) When the solids are hit with a hammer, W flattens out, X shatters into many pieces, Y is smashed into a powder, and Z is not affected. (c) When the solids are heated with a Bunsen burner, Y melts with some sublimation, but X, W, and Z do not melt. (d) In treatment with 6 MHNO3, X dissolves; there is no effect on W, Y, or Z. On the basis of these test results, identify the solids. Sample W Au PbS SiO2 Sample X: Au PbS Sample X: Au PbS SiO2 I, Sample Y: Au PbS SiO2 I2 Sample Z: Au PbS Au PbS SiO2 Sample Z: Au PbS SiO2
Answers
It has a metallic sheen that denotes the presence of lead sulphide and gold (Au). However the fact that it is unaffected by a hammer shows that quartz may also be present.
As sample W is an excellent electrical conductor and does not melt when heated with a Bunsen burner, the presented findings of the research indicate that it is gold (Au). When Sample X breaks into several pieces when struck with a hammer and dissolves in 6 MHNO3, it is lead sulphide (PbS). Since Sample Z is unaffected by hammer blows and does not melt when heated with a Bunsen burner, yet does not dissolve in 6 MHNO3, it is a combination of gold (Au), lead sulphide (PbS), and quartz (SiO2). It also exhibits a metallic shine, which denotes the presence of lead sulphide and gold. Nonetheless, the fact that a hammer has no effect on it shows that quartz may also be present.
learn more about gold (Au) here:
https://brainly.com/question/1673872
#SPJ4
Rutherfordium-261 has a half-life of 1.08 min. How long will it take for a sample of rutherfordium to lose one-third of its nuclei?
Answers
Answer:
\(t=1.712min\)
Explanation:
Hello!
In this case, since the radioactive decay equation is:
\(\frac{A}{A_0}=2^{-\frac{t}{t_{1/2} }\)
Whereas A stands for the remaining amount of this sample and A0 the initial one. In such a way, since the sample of rutherfordium is reduced to one-third of its nuclei, the following relationship is used:
\(A=\frac{1}{3} A_0\)
And we plug it in to get:
\(\frac{\frac{1}{3} A_0}{A_0}=2^{-\frac{t}{t_{1/2}} } \\\\\frac{1}{3}=2^{-\frac{t}{t_{1/2}} }\)
Now, as we know its half-life, we can compute the elapsed time for such loss:
\(log(\frac{1}{3})=log(2^{-\frac{t}{t_{1/2}} })\\\\log(\frac{1}{3})=-\frac{t}{t_{1/2}} }*log(2)\)
\(t=-\frac{log(\frac{1}{3})t_{1/2}}{log(2)} \\\\t=1.71min\)
Best regards!
Draw the structure of the tripeptide Gly-Gly-His. It has a role as a metabolite. Then determine the total charge of the tripeptide.
Answers
The molecular formula for tripeptide Gly-Gly-His is C10H15N5O4. The structure is attached below in the picture. It has a role as a metabolite.
A tripeptide is created by combining three amino acids, and it is then joined by two or even three peptide bonds. The order of the individual amino acids that make up a peptide dictates how it functions, just like with proteins. The simplest tripeptide is glycine. The most significant tripeptide, according to scientific studies, is glutathione (-L-Glutamyl-L-cysteinylglycine), which has a number of functions in a wide range of life forms.
To learn more about tripeptide click on the given link: https://brainly.com/question/28335208
#SPJ4

The diagram below shows the branching tree diagram for humans. The text box below it shows the set of derived shared characteristics for the branching tree.
A slanting, horizontal line is shown. On the extreme left, there is a label that says Common Ancestor. Along the slanting, horizontal line there are five dots labeled from left to right as 1, 2, 3, 4, and 5. There is one vertical line between each of the consecutive five dots. The lines are labeled from left to right as Perch, Frog, Pigeon, Rats, and Human. A text box below the branching tree diagram is labeled Derived Shared Characteristics. In the box it says from left to right, Terrestrial during all stages, Jaws, Walking on two legs, Mammary glands and hair, and Four limbs.
Look at the possible derived shared characteristics, shown in the text box. Think about where these should be placed along the branching tree diagram. From the text box, select a shared derived characteristic that humans and rats have. Explain why you think humans and rats share this characteristic.
Answers
Perch, frog, pigeon, and other species evolution would not have arisen prior to the derived shared trait of jaws and after the common ancestor.
How did the structure of the teeth of our ancestors change over time?The development of jaws in the bodies of our ancestors was a critical phase in the evolution of vertebrates, including humans. The earliest jawed vertebrates, called gnathostomes, descended from jawless fish and appeared in the fossil record around 420 million years ago.
What traits are similar between rats and people?Rats and people are both warm-blooded creatures that give birth to live children; both have similar organs, such as hearts and livers; both have comparable neurological systems; both employ comparable hormones to regulate body functions.
To know more about evolution visit:-
https://brainly.com/question/13484097
#SPJ1
How does the burning of fossil fuels contribute to global warming?
Answers
Answer: The burning of fossil fuels releases carbon dioxide, methane and other greenhouse gases into the atmosphere. These gases trap heat in the atmosphere, leading to a gradual increase in global average temperatures, known as global warming. This phenomenon has serious impacts on our environment and ecosystems, including extreme weather events and rising sea levels.
Please Help!
When the following equation is balanced, what is the coefficient in front of the
HCI?
HCI + CaCOS →
CaCl₂ + CO₂ + H₂O
o 1
o 2
o 4
o 3
Answers
CaCO3 (s) + 2HCl(aq) → CaCl2 (s) + H2O(aq) + CO2(g). The principle of mass conservation is essential for balancing chemical equations.
Which of the four reactions are they?Chemical Reaction TypesSynthesis processes reactions of decomposition.responses with only one substitution.reactions involving two replacements.
What is the class 10 double displacement reaction?Double displacement reactions often occur in aqueous solutions where there is an ion exchange and ion precipitation.For instance, a white precipitate containing barium sulphate forms right away when a solution containing barium chloride and sodium sulphate is combined.These are ionic-based reactions.
To know more about HCI visit:
https://brainly.com/question/15723116
#SPJ1
Put the following in order from SMALLEST to LARGEST: cell, nucleus, gene,
chromosome, DNA, organism,
Answers
Answer:
Here is the correct order (ascending order):
gene, DNA, chromosome, nucleus, cell, organism.
A gene is a segment of DNA that contains the instructions for building a protein or RNA molecule. DNA is the molecule that carries genetic information and makes up genes. A chromosome is a structure made of DNA and protein that carries genes. A nucleus is a membrane-bound organelle that contains chromosomes. A cell is the smallest structural and functional unit of living organisms, consisting of a nucleus (or nucleoid in prokaryotes) and cytoplasm. An organism is an individual living entity, such as a plant, animal, or bacterium, that consists of multiple cells.
A dose of medication was prescribed to be 350 microliters. Which of the following expresses that volume in centiliters, cL? (1 x 10-6 L = 1 microliters)
3.5 x 10-3 cL
3.5 x 105 cL
0.35 cL
3.5 x 10-2 cL
Answers
Answer:
0.035 cL
Explanation:
Given that,
A dose of medication was prescribed to be 350 microliters.
1 microliters = 10⁻⁶ L
350 microliters = 350 × 10⁻⁶ L
Also,
1 Liter = 100 centiliter
350 × 10⁻⁶ L = 350 × 10⁻⁶ × 100
= 0.035 cL
Hence, the required volume is 0.035 cL.
