Can regions that experience extreme low temperatures be located right
next to regions that experience very high temperatures?
Yes
No
Answers
Answer:No
Explanation:
Well yes but no, There cant be high tempreture that is 100 degrees and a low tempreture that is 20 degress, there has to be a mid inbetween both, they cant be next to eachother.
Related Questions
Which of the following best shows the basic three-step energy transformation for a battery-operated radio?
Responses
Acoustical → Mechanical → Electrical
Acoustical → Mechanical → Electrical
Mechanical → Acoustical → Chemical
Mechanical → Acoustical → Chemical
Chemical → Electrical → Acoustical
Chemical → Electrical → Acoustical
Electrical → Acoustical → Chemical
Answers
The energy transformation takes places in a battery operated radio is chemical energy to electrical energy then to sound energy or acoustical. Thus, fifth option is correct.
What is energy transformation?Energy can be transformed from one form to the other, For example, when we produce current from solar cells there occurs the transformation of light energy to electrical energy.
A battery contains two electrodes immersed in a molten electrolyte. When a potential difference is applied between these electrodes one of the electrode undergo reduction and the second one to oxidation.
The chemical energy produced from the electrolyte produce current and thus the energy is transformed to electrical energy. Using this electrical current the radio produces sound energy.
Therefore, the basic three- stage energy transformation for a battery operated radio is chemical energy to electrical energy then to sound energy or acoustical hence, fifth option is correct.
To find more about energy transformations, refer the link here:
https://brainly.com/question/8210521
#SPJ1
A student placed a straw into the water and blew bubbles into the water for 30 seconds. The pH of the glass of
tested again.
ater was
Use the pH scale below to determine the pH value of the water in this test. Record the value. Also, determine whether the
pH stayed the same, became more acidic, or became more alkaline compared to the first test.

Answers
Answer:
the water would most likely become more acidic.
Explanation:
this is due to carbon dioxide being slightly acidic, it dissolves into the water inturn decreasing the pH.
i also posted this on your question a minute ago before you posted it in the right catagory.
A chemist uses 0.25 L of 2.00 M H2SO4 to completely neutralize a 2.00 L of solution of NaOH. The balanced chemical equation of the reaction is given below.
Answers
1 mole of NaOH was neutralized by 0.5 moles of H2SO4, following the stoichiometry of the balanced chemical equation.
To determine the balanced chemical equation, we need to consider the reaction between sulfuric acid (H2SO4) and sodium hydroxide (NaOH). The reaction can be represented as follows:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
In this reaction, one molecule of sulfuric acid (H2SO4) reacts with two molecules of sodium hydroxide (NaOH) to produce one molecule of sodium sulfate (Na2SO4) and two molecules of water (H2O). The coefficients in front of the compounds represent the stoichiometric ratios between them.
Using this balanced equation, we can determine the molar ratio between H2SO4 and NaOH. From the equation, we can see that one mole of H2SO4 reacts with two moles of NaOH.
Given that the chemist used 0.25 L of 2.00 M H2SO4 and neutralized a solution of NaOH with a volume of 2.00 L, we can calculate the number of moles of H2SO4 and NaOH used.
Number of moles of H2SO4 = Volume (in liters) × Concentration (in M)
= 0.25 L × 2.00 M
= 0.5 moles
Since the molar ratio is 1:2 for H2SO4:NaOH, twice as many moles of NaOH would be required to neutralize the given amount of H2SO4.
Number of moles of NaOH = 2 × Number of moles of H2SO4
= 2 × 0.5 moles
= 1 mole
Therefore, 1 mole of NaOH was neutralized by 0.5 moles of H2SO4, following the stoichiometry of the balanced chemical equation.
learn more about stoichiometry here
https://brainly.com/question/28780091
#SPJ11
How many moles of carbon atoms are there in
5g of carbon?
Answers
Answer:
12 atoms of carbon
Explanation:
(6.023X10^23)/12 atoms of Carbon.
There are 0.42 moles of carbon atoms in 5g of carbon.
HOW TO CALCULATE NUMBER OF MOLES:The number of moles in a substance can be calculated by dividing the mass of the substance by its molar mass. That is;no. of moles = mass (g) ÷ molar mass (g/mol)
According to this question, the mass of carbon is 5g. The molar mass of carbon is 12g/mol.No. of moles = 5g ÷ 12g/mol
No. of moles = 0.42moles
Therefore, there are 0.42 moles of carbon atoms in 5g of carbon.
Learn more about number of moles at: https://brainly.com/question/24869996
co2 ammonia propane, butane and isobutane are all natural refrigerants
Answers
The use of natural refrigerants supports efforts to reduce greenhouse gas emissions and minimize the environmental impact of refrigeration and air conditioning systems.
Carbon Dioxide (CO2): CO2 is a natural refrigerant that has been used for many years. It is non-toxic, non-flammable, and has a low environmental impact. CO2 refrigeration systems are commonly used in commercial and industrial applications, especially in cascade systems and in supermarkets.
Ammonia (NH3): Ammonia is another widely used natural refrigerant. It has excellent thermodynamic properties, high energy efficiency, and is environmentally friendly. Ammonia is commonly used in industrial refrigeration systems, cold storage facilities, and large-scale applications.
Propane (C3H8): Propane, also known as R-290, is a hydrocarbon natural refrigerant. It is non-toxic, non-ozone depleting, and has a low global warming potential. Propane is primarily used in small-scale refrigeration and air conditioning applications, such as domestic refrigerators and freezers.
Butane (C4H10) and Isobutane (C4H10): Both butane and isobutane are hydrocarbon natural refrigerants. They have properties similar to propane and are also used in small-scale refrigeration and air conditioning systems. However, due to their higher flammability compared to propane, their use is typically more limited.
To learn more about environmental
https://brainly.com/question/1186120
#SPJ11
What reagent (or compound) causes the observed visual change in a positive Lucas test? A. Cr(VI) is reduced to Cr(ll) B. The alkyl halide product is insoluble in water C. The product hydrazone is insoluble. D. Ag() is reduced to Ag(0)
Answers
Hydrogen bonds cannot be broken by the energy released during the dissolution of solid alkyl halides with water. Because of their inability to dissolve the hydrogen atoms between water molecules, alkyl halides cannot withstand their attraction to one another. Insoluble in water, it continues to be.
Is hydrogen produced by any plants?In the process of photosynthesis, plants split water into nitrogen and oxygen with the help of sunlight, and then mix the generated hydrogen with atmospheric carbon dioxide to produce carbohydrates.
Do bodies benefit from hydrogen?Many of the factors implicated in the pathophysiology and etiology of the metabolic syndrome and the diseases it is associated with have been linked to molecular hydrogen's ability to attenuate peroxidation, enhance cellular function, and decrease chronic inflammation5.
To know more about hydrogen visit:
https://brainly.com/question/29130026
#SPJ4
how many hydrogen atoms are in one mole of CuNH4Cl3
Answers
Answer:
2.4088 x 10^24 atoms of H
Explanation:
+5
how many hydrogen atoms are in one mole of CuNH4Cl3
1 MOLECULE of CuNH4Cl3 contains 4 H atoms
1 molecules contain 2 X4 = 8 H atoms
a mole is 6.022 x 10^23 particles
1 mole of CuNH4Cl3 contains 6.022 x 10^23 molecules
so there are 4 x 6.022 x 10^23 H atoms = 24.088 x 10^23
=2.4088 x 10^24 atoms of H
2.4088 x 10^24 hydrogen atoms are in one mole of CuNH4Cl3.
What is hydrogen atom ?An atom of the chemical element hydrogen is known as a hydrogen atom. The Coulomb force holds a single positively charged proton and a single negatively charged electron to the nucleus of the electrically neutral atom. About 75% of the universe's total baryonic mass is made up of atomic hydrogen.
Many other applications exist for hydrogen. It is used in the chemical industry to produce cyclohexane and methanol, which are intermediates in the synthesis of polymers and medicines, as well as ammonia for agricultural fertilizer (the Haber process). In the course of the oil-refining process, it is also employed to remove sulphur from fuels.
1 molecule of CuNH4Cl3 contains 4 Hydrogen atoms
1 molecule contain 2 X 4 = 8 Hydrogen atoms
1 mole is 6.022 x 10^23 particles
1 mole of CuNH4Cl3 contains 6.022 x 10^23 molecules
Then, there are 4 x 6.022 x 10^23 H atoms
= 24.088 x 10^23
Thus, 2.4088 x 10^24 hydrogen atoms are in one mole of CuNH4Cl3.
To learn more about the hydrogen atom, follow the link;
https://brainly.com/question/29695801
#SPJ2
What volume do 78 grams of magnesium occupy?
Answers
Boyle's law foc endosed gases states that if the volume is kept constant, the pressure P and temperature T are related by the equinton P/T
=k Where k is a constant. If the tenperature is increasing at 3 kelvins per hour, what is the rate of charge of pressure when the temperature is 150keivins and the pressure is 300 pounds per squ inch? The rate of change of pressure is pounds per square inch per hous, (Simplity your answer) Find the relative rate of change of f(x)=100x−0.2x .2
.The relative rate of change of f(x) is
Answers
The rate of change of pressure is -900 pounds per square inch per hour.
According to Boyle's law, if the volume is kept constant, the pressure P and temperature T are inversely proportional, as expressed by the equation P/T = k, where k is a constant. To find the rate of change of pressure, we need to differentiate the equation with respect to time.
Since temperature is increasing at a rate of 3 kelvins per hour, we can substitute the given values into the equation.
Temperature (T) = 150 kelvins
Pressure (P) = 300 pounds per square inch
Differentiating the equation P/T = k with respect to time, we get:
dP/dt = (dP/dT) * (dT/dt)
Since the volume is constant, dP/dT is equal to -k. Therefore, we have:
dP/dt = -k * (dT/dt)
Substituting the given values:
dT/dt = 3 kelvins per hour
P = 300 pounds per square inch
We can simplify the equation to find the rate of change of pressure:
dP/dt = -k * (dT/dt) = -k * 3 = -3k
To determine the value of k, we need additional information. Since the problem statement does not provide a specific value for k, we cannot determine the exact rate of change of pressure.
However, we can express the rate of change of pressure as -900 pounds per square inch per hour, as k would cancel out when dividing the pressure by time.
Learn more about pressure.
brainly.com/question/14374618
#SPJ11
Solid lead nitrate is heated and forms solid lead oxide, gaseous nitrogen dioxide, and oxygen.
Answers
Solid lead nitrate is heated and forms solid lead oxide, gaseous nitrogen dioxide, and oxygen then it form of decomposition reaction
Lead nitrate is a white color inorganic powder with the chemical formula Pb ( NO₃)₂ when Lead nitrate decomposes it produces Lead oxide a yellow colored oxide of brown colored Nitrogen dioxide gas, and colorless Oxygen gas and also it gives yellow colors
When lead nitrate is heated it decomposes to form lead oxide, nitrogen dioxide and oxygen from the reaction, we can see that lead nitrate decomposes on heating and forms lead oxide, nitrogen dioxide and oxygen thus, lead nitrate on decomposition gives lead oxide, nitrogen dioxide and oxygen and the lead nitrate and when solid lead nitrate heated it decomposes to produce light yellow solid lead monoxide, reddish-brown nitrogen dioxide gas and colourless oxygen gas
Know more about lead nitrate
https://brainly.com/question/4588222
#SPJ1
a city council is debating between two potential water purification systems: reverse osmosis and ion exchange. cost is the primary criteria for the choice. which decision is the most likely result of this debate?(1 point)
Answers
It is likely that they would choose the ion exchange system as the most cost-effective option for water purification, provided it meets their specific water quality requirements.
Based on the student question, it appears that the city council is considering two water purification systems, reverse osmosis and ion exchange, with cost being the primary criterion for their decision.
In this scenario, the most likely result of the debate would be the selection of the water purification system with the lowest overall cost, taking into account both initial investment and ongoing operational expenses. To determine this, the city council would need to conduct a thorough cost analysis of each system.
Reverse osmosis is a process that uses pressure to force water through a semi-permeable membrane, removing contaminants and impurities. It is an effective method for purifying water, but the process can be energy-intensive and may require significant infrastructure investments, such as high-pressure pumps and specialized membranes. Additionally, ongoing costs can be high due to membrane replacement and energy usage.
for more such questions on water quality :
https://brainly.com/question/23717398
#SPJ11
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
Chlorine has 7 valence electrons. Hydrogen has 1 valence
electron. How many hydrogen atoms would form chemical
bond(s) with one chlorine atom
a 7
b1
C2
red a Hint?
d 4
Answers
This question involves the concepts of octet rule, valence shell, and valence electrons.
The number of hydrogen atoms that would form a chemical bond with one chlorine atom is "b) 1".
The octet rule states that in order for an atom to be stable, it must have 8 valence electrons in its valence shell. Therefore, the two elements always tend to form a bond in such a way that the resulting compound has eight valence electrons.
In a similar manner, chlorine which has seven valence electrons needs one more electron to complete its octet and become stable. On the other hand, a hydrogen atom has that one valence electron needed by chlorine.
Hence, the chlorine atom forms a chemical bond with one hydrogen atom to complete the octet for both the hydrogen and chlorine atoms.
Learn more about the octet rule here:
https://brainly.com/question/11982407?referrer=searchResults
The attached picture shows the octet rule in HCL.

identify the parts of the compound light microscope.
Answers
A compound light microscope consists of the following main parts:
Base: Supports the entire microscope and provides stability.
Body Tube: Connects the eyepiece to the objective lenses.
Arm: Supports the body tube and connects it to the base.
Nosepiece: Holds the objective lenses and allows them to rotate for easy magnification changes.
Objective Lenses: Provide magnification and are usually 4 lenses of different magnification powers (4x, 10x, 40x, 100x).
Condenser Lens: Focuses light onto the specimen and is adjustable for optimal illumination.
Diaphragm: Controls the amount of light that reaches the specimen.
Illumination Source: A bulb that provides light to the specimen.
Eyepiece: Magnifies the image and is usually 10x magnification.
Stage: Supports the slide and has clips to hold it in place.
Focus Knobs: Allow the user to adjust the focus by moving the stage and/or the body tube up and down.
Find out more about compound light microscope
brainly.com/question/30126710
#SPJ4
What is the classification of igneous rock which forms from lava on the earths surface?(Why is there no option for Geology for the subject thing btw)
Answers
Answer:
Extrusive and intrusive are the two major types of igneous rocks. On the Earth's surface, extrusive rocks are created from lava, which is magma that has erupted from the underground. Intrusive rocks are formed from magma within the planet's crust that cools and solidifies.
Explanation:
Yeet
True or False? the rate limiting step in a reaction is the slowest step in the reaction sequence.
Answers
The given statement is true. The rate limiting step in a reaction is the slowest step in the reaction sequence.
In chemical kinetics, the slowest step, sometimes referred to as the rate-determining step or rate-limiting step, frequently roughly determines the total pace of a reaction.
The reaction's slowest step is used to generate the rate equation. Writing rate equals the rate constant of the slowest step times the concentrations of the reactant or reactants raised to their reaction order is how you build up a rate equation.
2A + B → C + D using the following two stages, the first step is referred to as the rate-determining step of the reaction since the total rate of the reaction is equal to the rate of the slow first step. The rate of the reaction in the aforementioned equation depends on the rate constant k.
Learn more about rate limiting step here brainly.com/question/14923179
#SPJ4
Why do we need to use Roman Numerals in the names for Transition metals, Inner Transition metals and Group 14 metals in simple terms?
Answers
How much energy, in kj, is transferred between the system and surroundings when 250. 0 g of potassium fluoride is dissolved into water? the molecular mass of kf is 58. 10 g/mol. Give your answer as a positive number.
Answers
The energy, in kj, is transferred between the system and surroundings when 250. 0 g of potassium fluoride is dissolved into water, the molecular mass of kf is 58. 10 g/mol is 745 kJ per second thermodynamics
A thermodynamic device is the portion of the cosmos wherein observations are done, and the surroundings are the relaxation of the universe. The surroundings already has the entirety however the machine. We take into account that any tie desires to be broken energetically. The CO bond in acetone will therefore be broken with the aid of absorbing strength from the surrounding surroundings. presently, the acetone molecule's double bond between the CO atoms has a dissociation electricity of 745 kJ/mol. energy change will consequently be:Environmental strength is taken in at a fee of 745 kJ consistent with second (E = +745 kJ).The substance for which the chemical method KF stands is potassium fluoride. KF is the main supply of the fluoride ion for use in enterprise and chemistry, second best to hydrogen fluoride.A source of potassium is potassium fluoride, which is insoluble in water and used in oxygen-sensitive processes like metallic production.To learn more about thermodynamics, visit:
https://brainly.com/question/13669873
#SPJ4
Calculate the number of moles in a liquid sample of a substance that has a molar heat of fusion of 3.811 kJ/mol, if the sample releases 90.6 kJ when it freezes. Answer in units of mol Calculate the molar mass of the substance if the mass of the sample is 2022 g. Answer in units of g/mol.
Answers
Answer:
23.77mole,85.1g/mol
Explanation:
the moles of liquid is 23.77mole which is obtained by divide 90.6kj to 3.811kj/mol and molar mass is obtained from the formula of mole eg mole=mass/molar mass but mass is given and moles is obtained above.
When the molar mass of the significance of the mass of the sample is 2022 g is = 23.77mole, 85.1g/mol.
Calculation of Molar mass
When the molar mass of a chemical combination is defined as the mass of a sample of that compound separated by the amount of substance in that instance, measured in moles.
Although, The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the combination, which often change in mass due to the existence of isotopes.
When the moles of liquid is 23.77mole which is received by dividing 90.6kj to 3.811kj/mol and molar mass is acquired from the formula of mole eg mole is = mass/molar mass but the mass is given and moles is conveyed above.
Find more information about Molar mass here:
https://brainly.com/question/15476873
How many gram of olid alumnuim ulfied can be prepared by the reaction of 10. 0 gram of alumnium and 15. 0 gram of ulfur?how much of the non limiting
reactant in exce?
Answers
The mass of \(Al_{2} S_{3}\) produced is 15.616 grams.
The reaction taking place is as follows:
\(2Al+ 3S\) →\(Al_{2} S_{3} (s)\)
Moles of Al = mass/molar mass = 10.0g/27.0g/mol
= 0.370 mol
Moles of S = mass/molar mass = 15.0g/(32.065g/mol)
= 0.468 mol
Al and S reacts in the molar ratio of 2:3.
2 moles of Al reacts with 3 moles of S
0.370 moles of Al will react with S = (3/2)*0.370mol
= 0.555 mol
Similarly, 0.468 moles of S will react with Al = 2/3 *0.468mol
= 0.312 mol
Thus, Al is in excess and S is the limiting reactant (some of Al will be left over ,S will completely react)
So, moles of \(Al_{2} S_{3}\) produced=1/3*0.312 mol of S
= 0.104 mol
Mass of \(Al_{2} S_{3}\) produced = moles*molar mass of \(Al_{2} S_{3}\)
= 0.104mol*150.158g/mol
= 15.616 grams
To learn more about limiting reactants, here
https://brainly.com/question/14225536
#SPJ4
Draw the reagents needed to convert phenylacetonitrile (C6H5CH2CN) to the compound: C6H5CH2COC(CH3)3
Answers
The final product is C₆H₅CH₂COC(CH₃)₃, which is the desired compound.
To convert phenylacetonitrile (C₆H₅CH₂CN) to the compound C₆H₅CH₂COC(CH₃)₃, we can use the following reagents:
Lithium aluminum hydride (LiAlH₄): It is a strong reducing agent that can reduce the nitrile group (-CN) to a primary amine (-NH₂).
Acetone (CH₃COCH₃): It is a ketone that can react with the primary amine to form an imine intermediate.
Trimethylchlorosilane (Me₃SiCl): It is a silylating reagent that can react with the imine intermediate to form the desired compound C₆H₅CH₂COC(CH₃)₃.
The reaction sequence can be represented as follows:
Reduction of phenylacetonitrile:
C₆H₅CH₂CN + 4[H] → C₆H₅CH₂CH₂NH₂
Formation of imine intermediate:
C₆H₅CH₂CH₂NH₂ + CH₃COCH₃ → C₆H₅CH₂CH=NCH(CH₃)₂
Silylation reaction:
C₆H₅CH₂CH=NCH(CH₃)₂ + Me₃SiCl → C₆H₅CH₂COC(CH₃)₃ + HCl
The final product is C₆H₅CH₂COC(CH₃)₃, which is the desired compound.
To know more about phenylacetonitrile:
https://brainly.com/question/31756507
#SPJ4
9. What lab equipment is used to spread heat when heating to prevent glassware from cracking
breaking?
Answers

A sample of a gas occupies 6.00 liters at a temperature of 200 K. If the pressure remains constant and the temperature is raised to 600 K, the volume of gas sample would be 1.)18.0 L 2.)2.00 L 3.)3.00 L 4.)12.0 L
Answers
Answer:
18
Explanation:
The volume of the gas should be 1. 18.0 L
Calculation of the volume of the gas;Since A sample of a gas occupies 6.00 liters at a temperature of 200 K. If the pressure remains constant and the temperature is raised to 600 K
So here the volume of the gas should be
= 600 * 6 /200
= 18.0 L
hence, the first option is correct.
Learn more about temperature here: https://brainly.com/question/17895578
Create a visual model of an ionic substance (salt) dissolving in water and a covalent substance (sugar) dissolving in water.
Answers
Answer:
see image
Explanation:
let me know if you have any questions

32 N of Force. 37 N force. How strong is the net force and in which direction?
Answers
Explanation:
They are working against each other. That means you subtract.
32 - 37 = -5
You are moving 5 units to the left because 37 is negative which means it is moving left. The key word is left. In physics, you would get the same answer if you did it this way.
37 - 32 = 5
The 5 moves in the same direction as the 37
In the first step of glycolysis, the two reactions shown are coupled. Reaction 1: 3.3kcal/mol+ glucose +Pi⟶ glucose-6-phosphate +H2O
Reaction 2: ATP+H2O⟶ ADP+Pi+7.3 kcal/mol Answer the four questions about the first step of glycolysis. Is Reaction 2 spontaneous (favorable) or nonspontaneous (unfavorable)?
O spontaneous O nonspontaneous
Answers
Reaction 2
ATP + H2O ⟶ ADP + Pi + 7.3 kcal/mol is a spontaneous (favorable) reaction.
To determine whether a reaction is spontaneous or nonspontaneous, we can examine the change in free energy (ΔG) for the reaction. If ΔG is negative, the reaction is spontaneous, indicating that it can proceed independently without requiring an input of energy. If ΔG is positive, the reaction is nonspontaneous and requires an input of energy. In the given reaction, 7.3 kcal/mol is released as free energy. This implies that the reaction releases energy and is, therefore, exergonic. In other words, the products (ADP and Pi) have lower free energy than the reactants (ATP and H2O). Since the reaction releases energy and has a negative ΔG, it is considered spontaneous. Spontaneous reactions tend to occur naturally and do not require external energy sources. In the context of glycolysis, the coupling of Reaction 1
(glucose + Pi ⟶ glucose-6-phosphate + H2O)
with Reaction 2
(ATP + H2O ⟶ ADP + Pi + 7.3 kcal/mol)
Allows for the overall process of glucose phosphorylation to proceed. The energy released in spontaneous Reaction 2 drives the nonspontaneous Reaction 1, making the overall process energetically favorable.
To learn more about spontaneous reaction
brainly.com/question/31199175
#SPJ11
When Mg bonds with S, which of the following is true?a. Mg and S are in a "sea of electrons."b.Mg and S share two electrons.c. Mg gains two electrons, while S loses two electrons.d. Mg loses two electrons, while S gains two electrons.
Answers
Answer: Magnesium loses two electrons whilst sulfur gains tw
What is the formula of the Lonic compound formed by the elements lithium and oxygen
Answers
Answer:
Li2O
Explanation:
Lithium is an alkali metal that has three protons and three electrons. Oxygen is a nonmetallic gas that has eight protons and eight electrons. Lithium and oxygen bond to form an ionic compound.
3Li : 1s2 2s1......Li+
8O : 1s2 2s2 2p4.....O2-
FILL IN THE BLANK
Valence Electrons: Nonmetals v.s. Metals
Valence electrons in nonmetals occupy directional ___-orbitals.
Hardest substances known
Brittle, given enough ___
Metal valence electrons spread out into ___ s-orbitals.
Bonds do not "shatter"
Easily deformed (___)

Answers
Valence electrons in non-metal occupy directional p-orbitals.
Hardest substances known.Brittle, given enough force.Metal valence electrons spread out into spherical s-orbitals.
Bonds do not "shatter"Easily deformed (malleable).What are valence electrons?Valence electrons can be defined as the number of electrons that are present in the outermost shell of an atom. Also, valence electrons are typically used to determine whether an atom or group of elements found in a periodic table can bond with others.
This ultimately implies that, valence electrons is a property is typically used to determine the chemical properties of chemical elements.
What is a sublevel?A sublevel can be defined as an energy level that is associated with the electrons found outside the atomic nucleus.
The types of sublevel.In Chemistry, there are four (4) types of sublevel and these include the following:
I. s-orbital (sublevel): it has one (1) orbital i.e 1s.
II. p-orbital (sublevel): it has three (3) orbitals.
III. d-orbital (sublevel): it has five (5) orbitals.
IV. f-orbital (sublevel): it has seven (7) orbitals.
Read more on orbitals and electrons here: https://brainly.com/question/11404939
#SPJ1
fill in the blanks am giving brainliest and thanx
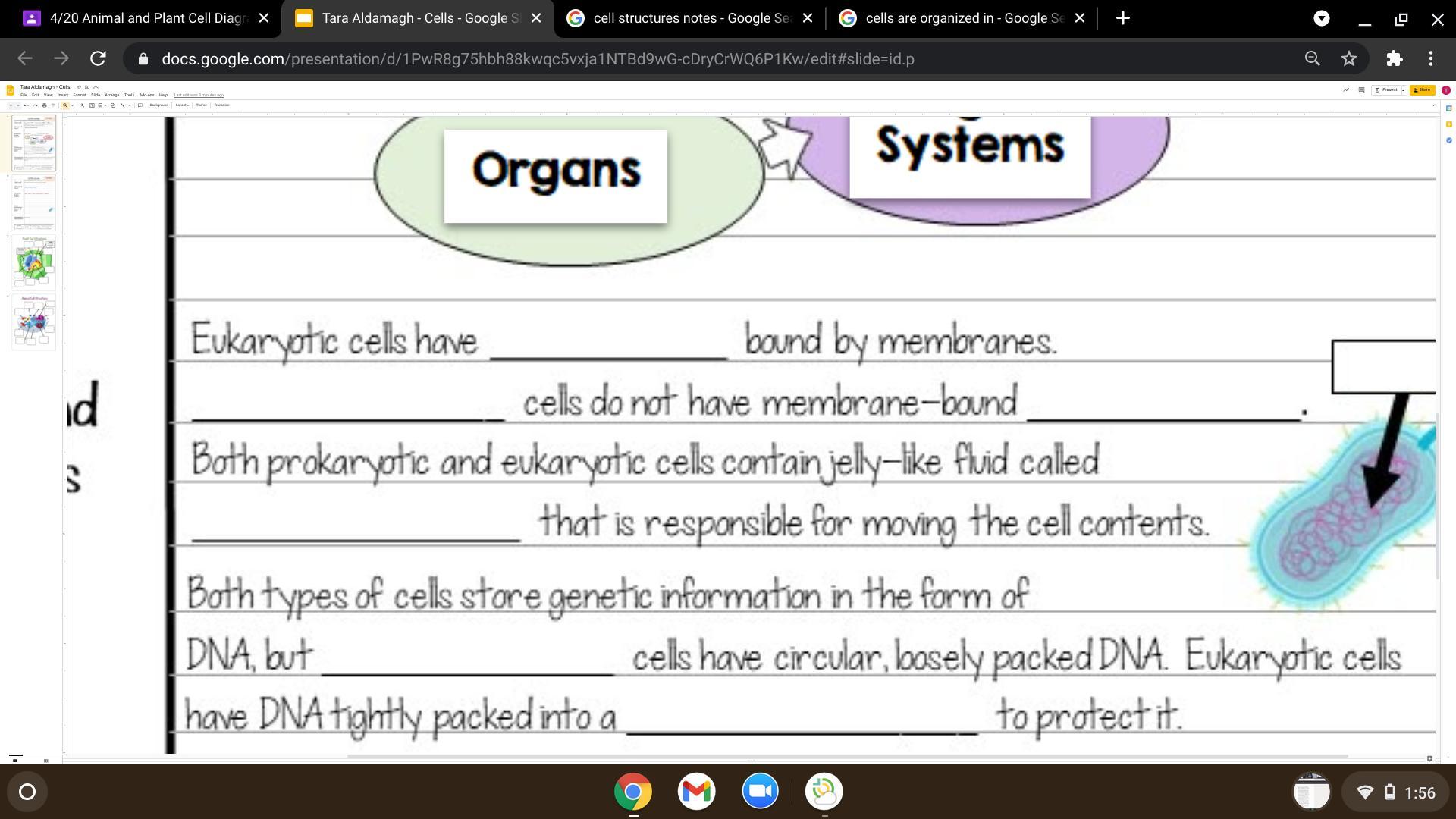
Answers
Answer:
Organelles, prokaryotic, organelles, cytoplasm, prokaryotic, nucleus
Explanation:
Organelles are membrane bound only in eukaryotic ccells.
Prokaryotic cells unlike eukaryotic cells, do not have membrane bound organlles.
Something that is found in both prokaryotic and eukaryotic cells is the cytoplasm, which is fluid that is inside the whole cell.
Unlike eukaryotic cells, prokaryotic cells have loose DNA, which floats around the cell quite literally.
The only way DNA is stored in eukaroyotic cells is in the nucelus, which is only found in eukaryotic cells, not prokaryotic.
Hope this helps ;)