Answers
Related Questions
Do Covalent bonds have weaker or stronger chemical bonds than Ionic bonds?
Answers
Ionic bonds are stronger than covalent bonds since they are of opposite charges. This makes them more attracted to each other, creating bonds that are harder to break. Covalent bonds don't have this and only occur to share electrons between atoms.
I hope this helps!
If 22.5 L of nitrogen at 748.3 mm Hg is changed to a pressure of 1294.6 mm Hg at constant
temperature. What is the new volume?
Answers
Answer:
it down their!!!!!!!!!!1
Explanation:
The new volume
=
23.2
L
Solar and wind energy are both intermittent resources that cannot be relied upon for a constant stream of energy production. Explain why developing better ways to store energy is an important part of making these energy sources more practical to use.
Answers
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers.
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers. Energy storage's inherent ability to offer backup power in the event of grid failure is a feature that both residential consumers and commercial owners find highly desirable.
To know more about energy, here:
https://brainly.com/question/1932868
#SPJ1
If [H3O^ + ]=1.7*10^ -8 M what is the pOH of the solution?
Answers
Answer: 6.23
Explanation:
1) solve for pH
pH=-log (H3O+) = - log 1.7 X 10^-8 =7.77
2) now do 14-pH = 14 -7.77=6.23
Every day a 6th grade class puts candy into bags to prepare for Valentine's Day. They put
7 pieces of candy in each bag. If they make 28 bags in 4 days, how many bags would
they make in 10 days?
Answers
They add “They put 7 pieces of candy in each bag to throw you off”
To find the answer just divide 28 bags by 4 days to get how many bags they make in 1 day. Take that number “7” and multiply it by 10 days. The answer is then 70.
A student carried out the synthesis described in this experiment. In the first step of the synthesis, the student combined 4.987 g of Fe(NH4)2(SO4)2·6H2O with 18.14 mL of 1.1 M H2C2O4. In the second step of the synthesis, the student added 20.5 mL of saturated K2C2O4, 21.2 mL of 3% H2O2, and 9.9 mL of 1.1 M H2C2O4 to the solid FeC2O4·2H2O produced in the first step. If H2C2O4 were the limiting reagent in the first step of the reaction, how many moles of FeC2O4·2H2O(s) would be created in the first step?
Answers
Answer:
\(n_{ FeC_2O_4\ 2H_2O}=0.020molFeC_2O_4\ 2H_2O\)
Explanation:
Hello.
In this case, since the chemical reaction for the first step is:
\(Fe(NH_4)_2(SO_4)_2\ 6H_2O + H_2C_2O_4 \rightarrow FeC_2O_4\ 2H_2O + H_2SO_4 + (NH_4)_2SO_4 + 4H_2O\)
Whereas we can see a 1:1 molar ratio between FeC2O4·2H2O(s) and H2C2O4, thus, we compute the moles of yielded FeC2O4·2H2O(s) in the first step as shown below:
\(n_{ FeC_2O_4\ 2H_2O}=0.01814L*1.1\frac{molH_2C_2O_4 }{L} *\frac{1molFeC_2O_4\ 2H_2O}{1molH_2C_2O_4} \\\\n_{ FeC_2O_4\ 2H_2O}=0.020molFeC_2O_4\ 2H_2O\)
Best regards.
Will give Brainliest!
A student titrates 25.0 mL of an unknown base with 0.10 M HCl. During the titration the pH is monitored and the collected data is recorded. These data are shown in the table below.
Volume
Added(mL) pH
0.0 11.13
5.0 9.86
10.0 9.44
12.5 9.26
15.0 9.08
20.0 8.66
22.0 8.39
24.0 7.88
25.0 5.28
26.0 2.70
28.0 2.22
30.0 2.00
35.0 1.70
37.5 1.61
40.0 1.52
45.0 1.40
50.0 1.30
a. Use the information provided to draw a titration curve showing the pH as a function of the volume of added HCl. Be certain to label your axes.
b. Identify the equivalence point on your graph and justify your selection of this particular point.
b. Use the data to determine the Kb value for the weak base. Be certain to show the mathematical steps you take to arrive at the answer. Report your final answer to the correct number of significant digits.
c. The student has three indicators that she could use for this experiment. The indicators (with their endpoints) are: Bromophenol Blue (3.0 – 4.6), Methyl Red (4.2 – 6.3), and phenolphthalein (8.3 – 10.0). Which indicator would be appropriate for this titration? Justify your selection.
e. Determine the (i) molarity and the (ii) % ionization of the original weak base solution (before titrating). Report your answers to the correct number of significant digits.
Answers
a. Titration Curve:
On the x-axis, label it as "Volume of HCl added (mL)"
On the y-axis, label it as "pH"
b. Equivalence Point:
The equivalence point is the point in the titration where the moles of acid (HCl) added are stoichiometrically equivalent to the moles of base (unknown base) present initially. In the given data, the equivalence point can be estimated to be around 25.0 mL of HCl added. This is where the pH drops dramatically from 7.88 to 5.28, indicating the neutralization of the base.
c. Calculation of Kb Value:
To determine the Kb value, we need to find the pOH at half-neutralization, where half the volume of the equivalent point has been reached. In this case, the half-neutralization volume is 12.5 mL (half of 25 mL).
From the data, we can observe that at 12.5 mL of HCl added, the pH is 9.26.
pOH = 14 - pH = 14 - 9.26 = 4.74
pOH = -log[OH-]
[OH-] = 10^(-pOH)
[OH-] = 10^(-4.74)
To find [OH-] in moles per liter (M), we need to convert mL to L.
[OH-] = 10^(-4.74) mol/L
Now, since we know that at the equivalence point, the concentration of the acid (HCl) is 0.10 M, we can use the stoichiometry of the reaction to determine the concentration of the base (unknown base).
From the balanced equation:
HCl + OH- → H2O + Cl-
1 mole of HCl reacts with 1 mole of OH-
0.10 M (HCl) = [OH-] M (unknown base)
Therefore, Kb = [OH-][unknown base] / [base]
Kb = (10^(-4.74) mol/L)(0.10 M) / (0.10 M - 10^(-4.74) M)
Simplify and calculate Kb.
c. Selection of Indicator:
Based on the given pKa ranges of the indicators, the indicator phenolphthalein (pKa range: 8.3 - 10.0) would be appropriate for this titration. The reason is that the pH at the equivalence point is expected to be around 7, which is well within the range of phenolphthalein's color change. Bromophenol Blue and Methyl Red have lower pKa values and would not be suitable for indicating the equivalence point in this particular titration.
d. Calculation of Molarity and % Ionization of the Weak Base Solution:
To calculate the molarity of the weak base solution, we can use the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
At the half-neutralization point, [A-] = [HA], and the pH is 9.26.
9.26 = pKa + log([A-]/[HA])
The pKa can be determined using the pOH at half-neutralization:
pKa = 14 - pOH = 14 - 4.74 = 9.26
9.26 = 9.26 + log([A-]/[HA])
log([A-]/[HA]) = 0
[A-]/[HA] = 10^0 = 1
Since [A-] = [HA], the concentration of the weak base (before titration) is equal to the concentration of its conjugate acid.
Therefore, the molarity of the weak base solution is 0.10 M.
To calculate the % ionization of the weak base, we can use the formula:
% Ionization = ([A-]/[HA]) × 100
% Ionization = (1/0.10) × 100
% Ionization = 1000%
Note: The % ionization may exceed 100% in cases where the concentration of the conjugate acid is very small compared to the concentration of the weak base.
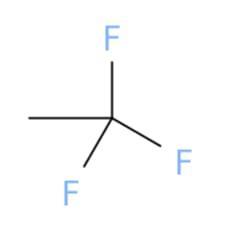
Select the correct structure that
corresponds to the name.
1,1,1-trifluoroethane

Answers
The correct chemical structure that corresponds to 1,1,1-trifluoroethane is (a).
What is 1,1,1-trifluoroethane?
A chemical structure is a spatial arrangement of atoms in a molecule. It determines the molecular geometry and when necessary the electronic chemistry as well .1,1,1-Trifluoroethane or simply known as trifluoroethane is Hydrofluorocarbon (HFC) compound that is colourless and highly inflammable gas with ether like odour. One method of preparation of 1,1,1-Trifluoroethane is by fluorination of 1-chloro-1,1-difluoroethane in the presence of hydrofluoric acid. The chemical formula for 1,1,1-Trifluoroethane is \(C__{2} } H_{3} F_{3}\). The high stability of it's chemical structure because of being heavier than air makes it a greenhouse gas with high infrared absorbent power. It can be used as a propellant or refrigerant and in cleaning of electrical equipments.
Learn more about 1,1,1-trifluoroethane here:
https://brainly.com/question/1390779
#SPJ1
what is the source of energy that is absorbed by the electrons to move from the ground state to excited state?
Answers
Answer:
I think an atom changes from a ground state to an excited state by taking on energy from its surroundings in a process called absorption. The electron absorbs the energy and jumps to a higher energy level. In the reverse process, emission, the electron returns to the ground state by releasing the extra energy it absorbed.
I hope this help you!:)
How does the burning of fossil fuels contribute to global warming?
Answers
Answer: The burning of fossil fuels releases carbon dioxide, methane and other greenhouse gases into the atmosphere. These gases trap heat in the atmosphere, leading to a gradual increase in global average temperatures, known as global warming. This phenomenon has serious impacts on our environment and ecosystems, including extreme weather events and rising sea levels.
results from liquid and plastic limit tests conducted on a soil are given here.liquid limit tests:number of blows, nmoisture content (%)1438.41636.52033.12827.0plastic limit tests: pl
Answers
The plasticity index of the soil is 17.2.
The plastic limit (PL) of the soil is given as 13.4%.
To find the liquid limit (LL) of the soil, we can use the given data from the liquid limit tests. The moisture content values are listed for four different numbers of blows: 38.4% for 14 blows, 36.5% for 16 blows, 33.1% for 20 blows, and 27.0% for 28 blows.
Assuming the plotted points have a linear relationship, we can use the two-point form of a straight line to determine the moisture content at 25 blows, ((27.0 - 33.1)/(28 - 20)) x (25 - 28) + 27.0 = 30.6%
Therefore, the liquid limit (LL) of the soil is 30.6%.
Finally, the plasticity index (PI) of the soil as:
PI = LL - PL
PI = 30.6 - 13.4
PI = 17.2
To know more about the plasticity index, here
brainly.com/question/17462239
#SPJ4
--The complete question is, Results from liquid and plastic limit tests conducted on a soil are given below.
Liquid limit tests:
Number of Blows: 14, 16, 20, 28
Moisture Content: 38.4, 36.5, 33.1 and 27.0 respectively for the number of blows mentioned.
Plastic limit tests: PL = 13.4%
a. Draw the flow curve and obtain the liquid limit.
b. What is the plasticity index of the soil?--

What state
of matter
exists in
area B?
A. gas
B. liquid
C. solid
Pressure
(atm)
61
6543210
0
50 100 150 200
Temperature (°C)
Answers
Considering the phase diagram, the state of matter that exists in area B is gas.
The correct option is A.
What is a phase diagram?A phase diagram is a graphical representation that shows the conditions of temperature and pressure at which different phases or states of a substance exist.
The axes of a phase diagram typically represent temperature (usually on the horizontal axis) and pressure (usually on the vertical axis). The diagram is divided into regions that correspond to different phases, and the lines separating these regions represent phase boundaries.
The point where three phase boundaries meet is known as the triple point, which represents the temperature and pressure at which all three phases can coexist in equilibrium.
Learn more about phase diagrams at: https://brainly.com/question/28097253
#SPJ1

10. Determine the frequency of light that has a wavelength of 400nm. c=X*v. C=3.0x108
Answers
Answer:
7.5 x 10^14 Hz
Explanation:
How many moles of a gas sample are in a 5.0 L container at 373 K and 203 kPa?
0.33 mole
0.66 mole
1.11 moles
3.05 moles
Answers
Answer:
0.33 moles.
Explanation:
Assuming the gas is ideal, we can solve this problem by using the following formula:
PV = nRTWhere:
P = 203 kPaV = 5.0 Ln = ?R = 8.314 kPa·L·mol⁻¹·K⁻¹T = 373 KWe input the data:
203 kPa * 5.0 L = n * 8.314 kPa·L·mol⁻¹·K⁻¹ * 373 Kn = 0.327 molThe answer is thus the first option, 0.33 moles.
Answer:
0.33 mole
Explanation:
A. If you have a Periodic Table that is NOT color coded, describe where to look on the Periodic Table to find elements which have similar chemical properties. B. Explain why they have similar chemical properties. C. Name three elements that have those similarities. Question 31 options:
Answers
Periodic table is a tabular chart of the chemical elements according to their atomic numbers so that elements with similar properties are in the same group (column).
The periodic table consists of elements arranged in a vertical (groups) and horizontal (periods) manner.
However, elements on the same group are known to have the same chemical properties because they possess the same number of valence electrons, hence, react chemically similar.
These elements have the same chemical properties because they contain the same number of valence electrons.
Example of elements with those similarities are chlorine, fluorine, iodine etc.
Learn more about periodic table at: https://brainly.com/question/11155928
#SPJ1
There are three states of matter: solid, liquid, and gas. is this true or false
Answers
Answer:
true
Explanation:
Solid, liquid and gases are the only three States of matter
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
This type of evidence is testimonial evidence. Statements that are made under oath
A. Trace Evidence
B. Indirect Evidence
C. Direct Evidence
D. Physical Evidence
Answers
Answer:
B
You are only trained as a Fry man kitchen helper in your restaurant. One day, one of the trained kitchen helpers assigned to the sauté station was absent. The Executive chef immediately assigned you to the sauté station by force.
What will you do considering that you have no training in that station?
1.
What causes convection currents to form in the ocean?
Differences in water density
Differences in water depths
Differences in water quality
2.
Which of the following is an example of heat transfer through convection?
Feeling heat from a campfire
smoke rising from a volcano
A metal spoon getting warm from being in hot water
3.
In which direction does heat always flow?
From a larger object to a smaller object
From a cooler object to a warmer object
From a smaller object to a larger object
From a warmer object to a cooler object
4.
Which of the following is the best description of convection?
Heat transfer between two objects that are touching
Heat transfer through movement in fluids
Heat transfer through empty space
80 points. 20 points each question.
Answers
1. Convection currents are caused by Differences in water density.
2. Feeling heat from a campfire is an example of heat transfer through convection.
3. Heat always flow from a warmer object to a cooler object.
4. The best description of convection is Heat transfer through movement in fluids.
Differential heating leads to convection currents. Warm, less dense, lighter material rises while cool, more dense, heavier material sinks. Convection currents are patterns of circulation that are produced by this movement in the Earth's mantle, oceans, and atmosphere.
The transfer of heat between two bodies by currents of moving gas or fluid is known as convective heat transfer. In free convection, air or water rises and is replaced by a cooler parcel of air or water as it moves away from the hot body.
Heat energy will always transfer from the warmer object to the cooler object under normal circumstances and in nature. Up until the two substances reach the same temperature, heat energy will transfer between them. The term "thermal equilibrium" describes this.
To learn more about heat transfer visit the link:
https://brainly.com/question/13433948?referrer=searchResults
#SPJ1
dentify the parts of the atom that are labeled in the diagram.
Answers
Answer:
Our current model of the atom can be broken down into three constituents parts – protons, neutron, and electrons. Each of these parts has an associated charge, with protons carrying a positive charge, electrons having a negative charge, and neutrons possessing no net charge.
Which gas is a greenhouse gas?
Oxygen
ammonia
Nitrogen gas
Water vapor
Answers
Answer:Nitrogen gas
Explanation:
I believe it is nitrogen correct me if i am wrong.
for the reaction at 400. k, . find the value of k for each of the following reactions at the same temperature:
Answers
The value of Kp for each of the reactions are 0.024 atm²; 6.4 atm⁻¹; and 1.681 x 10³ atm⁻⁴.
What is equilibrium constant?Equilibrium constant expression regarding the partial pressure is designated as Kp. Equilibrium constant Kp is same as the partial pressure of products divided by partial pressure of reactants and the partial pressure are increased with some power that is equal to the coefficient of the substance in balanced equation.
Pressure doesn’t affect the value of Kp, as concentration also doesn’t affect the value of Kc. A raise in pressure causes equilibrium to shift in favor of the direction with the fewer moles, so then the pressure reduces. The partial pressure ratio of reactant to products remains the same so Kp doesn’t change.
In this case, value of Kp for each of the following reactions are:
(i) 2NH₃ ⇌ N₂ +3H₂;
Kp = [H₂]³[N₂] / [NH₃]² = [ [NH₃]² / [N₂][H₂]³ ]⁻¹ = (41)⁻¹ = 1 / 41 = 0.024 atm²
(ii) 1/2N₂ + 3/2H₂ ⇌ NH₃;
Kp = [NH₃] / [H₂]³⁻² [N₂]¹⁻² = [ [NH₃]² / [H₂]³ [N₂] ]¹⁻² = (41)¹⁻² = 6.4 atm⁻¹
(iii) 2N₂ + 6H₂ ⇌ 4NH₃.
Kp = [NH₃]⁴ / [N₂]² [H₂]⁶ = [ [NH₃]² / [N₂][H₂]³ ]²= (41)² = 1.681 × 10³ atm⁻⁴
Learn more about equilibrium constant at: https://brainly.com/question/10038290
#SPJ4
Although part of your question is missing, you might be referring to this full question: For the reaction N₂ + 3H₂ ⇌ 2NH₃. At 400 K, Kp = 41 atm⁻². Find the value of Kp for each of the following reactions at the same temperature:
(i) 2NH₃ ⇌ N₂ +3H₂;
(ii) 1/2N₂ + 3/2H₂ ⇌ NH₃;
(iii) 2N₂ + 6H₂ ⇌ 4NH₃.
5. For the following reaction:
I2 (g) + H2 (g)>2HI (g)
If the amount consumed from (I2) according to the above reaction is (50.8g) and the actual yield of HI is (40g) then the percentage yield of (HI) is equal to :
Answers
1.25 is the closest to 1.04 or not I want to answer please. I think it's true, but I want to prove it scientifically, please.
Answers
Answer:
false because if you round both of them
When fluorine gas is put into contact with calcium metal at high temperatures calcium fluoride powder is formed?; What is the chemical equation for calcium and fluorine?; What is the formula for fluorine gas?; How do you balance calcium fluoride?
Answers
When fluorine gas is put into contact with calcium metal at high temperatures, calcium fluoride (CaF2) is formed.
The chemical equation for the reaction between calcium and fluorine is:
Ca + F2 -> CaF2
The formula for fluorine gas is F2.
To balance the equation for the reaction between calcium and fluorine to form calcium fluoride, you need to ensure that there are the same number of atoms of each element on both sides of the arrow. The balanced equation is:
Ca + F2 -> CaF2
There is 1 atom of calcium on the left side and 1 atom of calcium on the right side, so the equation is already balanced with respect to calcium. There are 2 atoms of fluorine on the left side and 2 atoms of fluorine on the right side, so the equation is also balanced with respect to fluorine.
Therefore, the balanced equation for the reaction between calcium and fluorine to form calcium fluoride is:
Ca + F2 -> CaF2
A chemical equation is a written representation of a chemical reaction that shows the reactants, products, and their coefficients. The reactants are the substances that are present at the beginning of the reaction, and the products are the substances that are produced as a result of the reaction. The coefficients are the numbers that are placed in front of the reactants and products to indicate the relative amounts of each substance involved in the reaction.
Learn more about chemical equation, here https://brainly.com/question/28294176
#SPJ4
1. Several solids, liquids, and gases can be found in your home. List three examples of each. (9 points) Think about where solids, liquids, and gases might be found in your refrigerator, bathroom, or basement/garage.
2. What states of matter exist within the human body? What state of matter do you think your body is mostly made up of? Why? (4 points) Think about whether the body contains solids, liquids, or gases. Which of the three would you be most likely to find?
3. Your blood contains many dissolved solids. What do you think could be done if you needed to remove the water from a sample of blood in order to study the solids that remained? (4 points) Think about what processes remove water from watery foods, solutions, or objects.
4. Your body contains a considerable amount of dissolved metal ions. Based on what you know about food and nutrition, list at least three metals you think could be found within the human body. (3 points) Refer to the periodic table — do any of the metal element names seem familiar? (Think about the ingredients list printed on food labels.)
Answers
1. Examples of solids, liquids, and gases found in a home
Solids: books, furniture, toys
Liquids: water, juice, shampoo
Gases: air, natural gas, propane
2. The human body contains solids, liquids, and gases. Solids include bones, muscles, and organs. Liquids include blood, saliva, and urine. Gases include air in the lungs and dissolved gases in the bloodstream. The body is mostly made up of liquids, as they make up a large percentage of its overall volume.
3. If you needed to remove the water from a sample of blood to study the solids that remained, you could use a process such as evaporation or freeze-drying. Evaporation involves heating the sample to allow the water to evaporate, leaving behind the solids. Freeze-drying involves freezing the sample and then removing the water under vacuum, leaving behind a dry solid.
4. Some metals that could be found within the human body include iron, zinc, and copper. These metals are commonly found in foods such as meat, seafood, nuts, and whole grains. Other metals such as calcium, magnesium, and potassium are also important for the body and are found in a variety of foods.
To know more about solids here
https://brainly.com/question/21500863
#SPJ1
Which compound has the most nonbonding electrons?
A. Butene
B. Octane
C. Nitrogen monoxide
D. Water
E. Carbon dioxide
Answers
Answer:
d water I believe I'm sorry if it's wrot
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
To find the order of a reaction with respect to one reactant, you will monitor the as the of . is changed.
Answers
The order of reaction is defined as the power to which the concentration of the reactants are raised in the rate equation of the reaction.
The order of reaction can be used to determine how a particular reactant affects the reaction. In order to find the order of a reaction with respect to a particular reactant, the concentration of the reactant is changed while keeping the concentration of other reactants constant. The rate of reaction is then measured and compared with the rate of reaction when the concentration of the reactant is not changed.The order of reaction with respect to a reactant can be determined using the following method:First, select a reactant whose order needs to be determined and change its concentration while keeping the concentration of other reactants constant. For example, if we want to find the order of reaction with respect to reactant A, we will change the concentration of A and keep the concentration of reactant B constant.Second, measure the rate of reaction at different concentrations of the reactant A. The rate of reaction can be measured by any suitable method such as change in color, pH, or by measuring the amount of product formed with time. A graph is plotted with rate of reaction on the y-axis and concentration of reactant A on the x-axis. The graph should be a straight line.Third, if the graph is a straight line passing through the origin, the order of reaction with respect to reactant A is one. If the graph is a straight line but does not pass through the origin, the order of reaction with respect to reactant A is two. If the graph is not a straight line, the order of reaction with respect to reactant A is either zero or fractional.For such more question on concentration
https://brainly.com/question/17206790
#SPJ8
Determine the mass of H2O produced if 1.75g of AL(CH)3 reacts with 2.00g of H2SO4 if H2SO4 is a limitating reagent
Answers
Answer: We must first compute the quantity of H2O that can result from the reaction of AL(CH)3 and H2SO4 in order to determine the mass of H2O produced.
Explanation:
Let's begin by formulating the reaction's balanced chemical equation:
Al2(SO4)3 + 6 H2O + 2 CH4 = 2 AL(CH)3 + 3 H2SO4
According to the equation, 2 moles of AL(CH)3 and 3 moles of H2SO4 combine to form 6 moles of H2O.
1.75 g of AL(CH)3 is equal to: 1.75 g / 78.0 g/mol = 0.0224 mol of AL(CH)3 as 1 mole of AL(CH)3 has a molar mass of roughly 78.0 g/mol.
2.00 g of H2SO4 is equal to: 2.00 g / 98.1 g/mol = 0.0204 mol of H2SO4 as 1 mole of H2SO4 has a molar mass of roughly 98.1 g/mol.
In order to calculate the amount of H2O produced, we must now identify the limiting reagent.
We contrast the moles of AL(CH)3 and H2SO4 in use. AL(CH)3 and H2SO4 have a stoichiometric ratio of 2 to 3. As a result, 3 moles of H2SO4 are required for every 2 moles of AL(CH).
The equation states that 2 moles of AL(CH)3 will yield 6 moles of water. Therefore, we can calculate the moles of H2O created for 0.0224 mol of AL(CH)3 as follows: 0.0224 mol AL(CH)3 (6 mol H2O / 2 mol AL(CH)3) = 0.0672 mol H2O
Finally, we may use the molar mass of H2O to convert its moles to grams. H2O has a molar mass of about 18.0 g/mol:
18.0 g/mol x 0.0672 mol H2O = 1.21 g H2O
As a result, the mass of H2O generated is roughly 1.21 grams if H2SO4 is the limiting reagent.