Answers
Answer:
2nd or 3rd dyfudhfkfhxhxhxjxjcjcifid
Related Questions
Isoctane of molecular formula(C8H18 ) is one of the principal constituents of car fuel. 20g of isoctane is burned:
a) Write the equation for the complete combustion of isoctane.
b) Calculate the number of moles of oxygen consumed.
Answers
The number of moles of oxygen consumed in the combustion of 20g of isoctane is 4.375 moles.
a) The equation for the complete combustion of isoctane is:
\(C_8H_{18} + 25O_2 --> 8CO_2 + 9H_2O\)
b) To calculate the number of moles of oxygen consumed, we first need to find the number of moles of isoctane used.
The molar mass of isoctane (\(C_8H_{18}\)) can be calculated as:
(8 x 12.01 g/mol) + (18 x 1.01 g/mol) = 114.16 g/mol
So, 20g of isoctane is equal to:
20 g ÷ 114.16 g/mol = 0.175 moles
According to the balanced chemical equation, 25 moles of oxygen are required to completely combust 1 mole of isoctane. Therefore, for 0.175 moles of isoctane, the amount of oxygen consumed can be calculated as:
0.175 moles x 25 moles of \(O_2\)/1 mole of isoctane = 4.375 moles of \(O_2\)
To learn more about isoctane click here https://brainly.com/question/29526251
#SPJ11
To calculate the number of moles of oxygen consumed, we need to use the balanced equation to determine the mole ratio between isoctane and oxygen.
a) The equation for the complete combustion of isoctane is:
C8H18 + 12.5O2 → 8CO2 + 9H2O
b) From the equation above, we can see that 1 mole of isooctane reacts with 12.5 moles of oxygen.
First, we need to calculate the number of moles of isoctane in 20g:
Number of moles of isoctane = mass / molar mass
Number of moles of isoctane = 20g / 114.23 g/mol
Number of moles of isoctane = 0.175 moles
Using the mole ratio, we can then calculate the number of moles of oxygen consumed:
Number of moles of oxygen consumed = number of moles of isoctane × mole ratio of oxygen to isoctane
Number of moles of oxygen consumed = 0.175 moles × 12.5
Number of moles of oxygen consumed = 2.1875 moles
Therefore, 2.1875 moles of oxygen are consumed during the combustion of 20g of isoctane.
Learn more about moles here:
https://brainly.com/question/31597231
#SPJ11
what is indepent and dependent variables
Answers
Answer:
The independent variable is the cause. Its value is independent of other variables in your study.
The dependent variable is the effect. Its value depends on changes in the independent variable.
Ex: The ice cube melts on the stove.
Dependent variable: Ice Cube
Independent variable: heat of stove
Answer:
In an experiment, the IV(independent variable) is a variable that is changed to see how it affects something else, and the DV (dependent variable)is a variable that is being measured/observed.
hope this help!
please mark as brainiest <3
Which property of the isotopes must be different?
A) The atomic number
B) The electric charge
C) The element name
D) The mass number
Answers
Which of these accurately expresses the distance of the Earth to the Sun in scientific notation in kilometers
O 1.5 x 108 km
150 million km
150 x 106 km
O 93 million km
Answers
Answer:
150million km
Explanation:
hope it helps
true or false. a hot plate is the only heat source available in the lab room to heat the hydrate in a crucible at least 2 times for 10-15 minutes at medium-high setting.
Answers
The given statement "A hot plate is the only heat source available in the lab room to heat the hydrate in a crucible at least 2 times for 10-15 minutes at medium-high setting" is true because the water in hydrate can be removed by heating.
The crucible is the type of the laboratory glassware that is designed to melt or to burn the solid chemicals over the burner. Crucible is made from the heat resistant ceramic or the metal. The hot plates are the laboratory tools that is used for the uniformly heat the samples. The hot plates are available with Varity of the number of the different heating top styles.
Thus, the hot plate is the heating tool to heat the hydrate in the crucible.
To learn more about crucible here
https://brainly.com/question/29220811
#SPJ4
What is the velocity of a wave with a wavelength of 0.03 m and a frequency of
120 Hz?
A. 0.28 m/s
B. 4000 m/s
C. 3.6 m/s
D. 2.5x 10-4 m/s
Answers
Answer:
A
Explanation:
Got a calculation of 8.05297
Answer:
C. 3.6 m/s
Explanation:
I just took a test on a-pe-x and it was correct! :)
What mass of hydrogen will react with 84g of N2
Answers
The balanced chemical equation for this reaction is:
N2 + 3H2 → 2NH3
This means that one mole of N2 reacts with three moles of H2 to produce two moles NH3.
To find out how many moles of N₂ are present in 84 g, we can use its molar mass which is about 28 g/mol.
Number of Moles = Given Mass / Molar Mass
= 84 g /28 g/mol
= 3 mol
From the balanced chemical equation above, we know that it takes three moles of H₂ to react with one mole N₂. Therefore,
1 mol N₂ : 3 mol H₂
Using these ratios, we can calculate the number of moles needed for Hydrogen.
Moles H₁= Ratio * given amount
= (3/1)* Number_of_Moles_Nitrogen
=(3/1)* (3mol)
=9mol
Now that you have calculated how many moles are needed based on stoichiometry calculation, you can now calculate the mass using :
Mass= Number Of Moles * Molar Mass
=9mol* (about) 2g/mol
≈18 g
Therefore, approximately **18 grams** of hydrogen will react with **84 grams**of Nitrogen.
whats the molar mass of CO
Answers
hope that helped
Answer:
28.01
Explanation:
this is the molar mass
The gas in an aerosol container is at a pressure of 3. 50 atm at 24. 0°c. The caution on the container warns against storing it at temperatures above 95°c. What would the gas pressure in the container be at 95°c?.
Answers
The pressure of the gas in the aerosol container at 95°C would be 7.47 atm. Therefore, storing it at temperatures above 95°C is dangerous. The gas pressure in the container depends on its temperature and volume. The pressure of the gas in the aerosol container at 95°C would be 4.31 atm. Storing it at temperatures above 95°C is dangerous.
Given, The pressure of the gas in an aerosol container is 3.50 atm. The temperature of the gas is 24.0°C.The temperature above which it should not be stored is 95°C.The relationship between the pressure, volume, and temperature of a gas is given by the ideal gas law PV = nRT where P is the pressure, V is the volume, n is the number of moles, R is the universal gas constant, and T is the temperature. The given pressure of the gas is 3.50 atm and the temperature is 24.0°C. Hence, we need to convert it to Kelvin as follows:24.0°C + 273 = 297KThe temperature at which the aerosol container should not be stored is 95°C. Therefore, we need to convert this temperature into Kelvin as follows:95°C + 273 = 368KNow we can calculate the pressure of the gas at the given temperature using the ideal gas law: n, V, and R are constants, so we can write: P₁/T₁ = P₂/T₂ Thus, 3.50/297 = P₂/368Solving for P₂, we get: P₂ = 3.50 × 368/297P₂ = 4.31 atm Therefore, the pressure of the gas in the aerosol container at 95°C would be 4.31 atm. Storing it at temperatures above 95°C is dangerous.
To know more about temperatures visit :
https://brainly.com/question/14532989
#SPJ11
What is the empirical formula of a compound containing 83% potassium and 17% oxygen?
Answers
The empirical formula of the compound containing 83% potassium and 17% oxygen is K₂O.
What is the empirical formula of the compound with 83% potassium and 17% oxygen?The empirical formula represents the simplest whole-number ratio of atoms present in a compound. To determine the empirical formula, we need to convert the given percentages of potassium and oxygen into mole ratios.
Assuming we have 100 grams of the compound, we would have 83 grams of potassium and 17 grams of oxygen. To find the moles of each element, we divide the mass by their respective molar masses. The molar mass of potassium (K) is 39.10 g/mol, and the molar mass of oxygen (O) is 16.00 g/mol.
Converting the masses to moles, we find that we have approximately 2.12 moles of potassium and 1.06 moles of oxygen. To obtain the empirical formula, we divide the moles by the smallest number of moles, which is 1.06. This gives us a ratio of approximately 2:1.
Therefore, the empirical formula of the compound is K₂O, indicating that it contains two potassium atoms for every oxygen atom.
Learn more about empirical formula
brainly.com/question/32125056
#SPJ11
difference between a compound and a mixture , Simple and short answer please!!
Answers
Some weather conditions are shown.
Weather Conditions
• Cold front moving in behind warm, moist air
• Heavy, constant winds
• Sudden hail
• Unstable atmosphere
Which type of severe event is MOST likely to occur during these weather conditions?
Answers
Answer:
Tornado
Explanation:
This is because, tornados are heavy constant winds that are blowing around all over causing an unstable atmosphere. Sometimes the weather will change drastically like causing hail, and cold fronts moving in. This is exactly what matches the conditions you show! :)
Hope this helps! Please mark as brainliest!
Why is it important to clean debris from the external surface of the handpiece prior to sterilization?
Answers
It important to clean debris from the external surface of the handpiece prior to sterilization because "the autoclave is capable of vaporizing mercury through amalgam restorations that have been removed."
Chemical vapor sterilizers, dry heat, as well as autoclaves are all appropriate sterilization techniques. Dental handpieces should not be used with ethylene oxide gas.
It is crucial to clean our dental handpieces with a mild detergent that would be germicidal in nature but isn't a disinfectant before sterilizing them. It is also possible to disinfect the handpiece's exterior with isopropyl alcohol.
Therefore, the autoclave is capable of vaporizing mercury through amalgam restorations that have been removed."
To know more about handpiece prior
brainly.com/question/7335064
#SPJ4
Potassium chlorate decomposes according the following BALANCED equation.
2 KClO3 → 2 KCl + 3O2
How many grams of oxygen gas would be produced if 33.0 g of potassium chlorate react?
Answers
Answer:
12.9 g O₂
Explanation:
To find the mass of oxygen gas produced, you need to (1) convert grams KClO₃ to moles KClO₃ (via molar mass from periodic table values), then (2) convert moles KClO₃ to moles O₂ (via mole-to-mole ratio from reaction coefficients), and then (3) convert moles O₂ to grams O₂ (via molar mass). It is important to arrange the conversions/ratios in a way that allows for the cancellation of units (the desired unit should be in the numerator). The final answer should have 3 sig figs to match the given value (33.0 g).
Molar Mass (KClO₃): 39.098 g/mol + 35.45 g/mol + 3(15.998 g/mol)
Molar Mass (KClO₃): 122.542 g/mol
2 KClO₃ ---> 2 KCl + 3 O₂
Molar Mass (O₂): 2(15.998 g/mol)
Molar Mass (O₂): 31.996 g/mol
33.0 g KClO₃ 1 mole 3 moles O₂ 31.996 g
-------------------- x ------------------- x ----------------------- x ------------------ =
122.542 g 2 moles KClO₃ 1 mole
= 12.9 g O₂
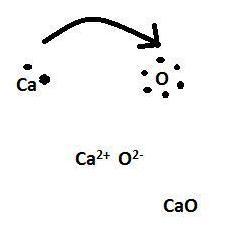
the correct lewis dot structure for CaO?
Answers
Answer:
Here you go.
Explanation:
Hopefully it is not confusing.
why metals are not often used to
make clothes
Answers
Answer:
They would be to heavy and you would be really stiff
Explanation:
Imagine walking in a suit made of iron or tin
You would feel like the Tin Man
You would be stiff
Metals are hard and heavy
A car accelerates from 0 to 72 km/hour in 6.0 seconds. What is the car's acceleration?
Answers
The acceleration of the car is equal to 3.33 m/s² with a velocity of 72 Km/hr.
What is acceleration?Acceleration can be demonstrated as the rate of change of velocity over time. The acceleration can be explained as vector quantity due to both magnitude and direction. Acceleration is defined as the 2nd derivative of the position of the object w.r.t. time.
According to Newton's 2nd law of motion, the force exerted is equal to the mass times of acceleration of an object.
a = F/m
or, a = dv/dt
Given, the final velocity of the car, v = 72 Km/hr
v = 72 × 1000/3600 m/s
v = 20 m/s
The time is taken by car to travel, t = 20 s
The acceleration of the car, a = (20 - 0)/(6 - 0)
a = 3.33 m/s²
Therefore, the acceleration of the car is 3.33 m/s².
Learn more about acceleration, here:
brainly.com/question/3046924
#SPJ5
17. Interpret each chemical formula Mn₂(CO3)3. Determine how many atoms of each
element make up the compound.
Answers
The chemical formula Mn₂(CO₃)₃ represents a compound composed of manganese (Mn) and carbonate (CO₃) ions and contains 2 manganese atoms, 9 carbon atoms, and 9 oxygen atoms.
Understanding the Component of a Chemical FormulaTo determine the number of atoms of each element in the compound, we need to break down the formula and analyze the subscripts.
Breaking down the formula
- Mn₂ indicates that there are two manganese atoms in the compound.
- (CO₃)₃ indicates that there are three carbonate ions in the compound. Each carbonate ion consists of one carbon atom (C) and three oxygen atoms (O).
Analyzing the carbonate ion
Since there are three carbonate ions in the compound, we need to multiply the number of atoms in each ion by three:
- There are three carbon atoms (C) in each carbonate ion, so in total, there are 3 x 3 = 9 carbon atoms.
- There are three oxygen atoms (O) in each carbonate ion, so in total, there are 3 x 3 = 9 oxygen atoms.
Summing up the atoms
- Manganese (Mn): 2 atoms
- Carbon (C): 9 atoms
- Oxygen (O): 9 atoms
Therefore, the compound Mn₂(CO₃)₃ contains 2 manganese atoms, 9 carbon atoms, and 9 oxygen atoms.
Learn more about chemical formula here:
https://brainly.com/question/11574373
#SPJ1
A motorcycle starts at rest and moves a distance of 460m .
If it has constant acceleration of 4m55 m/s2 what is its final velocity
Answers
Answer:
The final velocity of the motorcycle is 64.7 m/s
Note: Since the value for the acceleration of the motorcycle is not clear, it is assumed to be 4.55 m/s² in the calculation
Explanation:
Using the equation of motion that contains all the given values in the question: v² = u² + 2as
where v is final velocity; u is initial velocity; a is acceleration; s is horizontal distance
v = ?, u = 0 (since the motorcycle starts from rest), a = 4.55 m/s², s = 460 m
v² = 0² + 2 * 4.55 * 460
v² = 4186
take square root of both sides
v = 64.7 m/s
Therefore, the final velocity of the motorcycle is 64.7 m/s
Click on the reset button. Expand the Net Charge' and 'Mass Number' menus by clicking the green + on the right side of the boxes. Change the numbers of protons, neutrons, and electrons and observe the effects of these changes on the net charge and mass number of the element. Answer the following questions after you've Investigated what happens. 15. What variables are you manipulating in this exercise? Identify a symbol to represent each variable.
Answers
Changing the numbers of protons, neutrons, and electrons can have a significant impact on the net charge and mass number of an element.
In this exercise, there are three variables being manipulated. They are the numbers of protons, neutrons, and electrons. The symbol to represent each variable are:Protons - represented by the symbol P.Neutrons - represented by the symbol N.Electrons - represented by the symbol E.The number of protons is equal to the atomic number of an element, which is a unique identifier for that element. The number of neutrons and electrons can vary, which results in the creation of different isotopes of the same element. Isotopes are atoms of the same element that have different numbers of neutrons but the same number of protons.The net charge of an atom is determined by the difference between the number of protons and electrons. If there are more protons than electrons, the net charge is positive. If there are more electrons than protons, the net charge is negative. If the number of protons and electrons are the same, the net charge is neutral.The mass number of an atom is determined by the total number of protons and neutrons. Electrons have a negligible mass and are not included in the calculation of the mass number.
for more questions on mass
https://brainly.com/question/24191825
#SPJ8
if i take 2 tables two times per day with 300 tables how many days will my prescription last
Answers
Answer:
150
Explanation:
300 divided by 2
a reactant decomposes with a half-life of 103 103 s when its initial concentration is 0.154 0.154 m. when the initial concentration is 0.664 0.664 m, this same reactant decomposes with the same half-life of 103 103 s. what is the order of the reaction? 2 0 1 what is the value and unit of the rate constant for this reaction?
Answers
The order of reaction is 1 and the value of the rate constant is 6.74*10^-5s^-1.What is the order of the reaction? To solve this question we have to check the half-life of a chemical reaction at different concentrations.
It is given in the question that when the initial concentration of reactant is 0.154m, it has half-life of 103s. Similarly, when initial concentration is 0.664m, it has half-life of 103s. To find the order of reaction, we have to use the equation for half-life of reaction. The equation for half-life of reaction is given as:\[\frac{t_{1/2} }{2}=\frac{1}{k}\frac{1}{[A]_{0}}\]Where, t1/2 is half-life of the reaction,[A]0 is the initial concentration of the reactant and k is the rate constant.
Putting the values in equation: When [A]0 = 0.154m and t1/2 = 103s, we get:\[\frac{103}{2}= \frac{1}{k} \frac{1}{0.154}\] Multiplying both sides with 0.154k:\[k*\frac{103}{2}*0.154 = 1\]When [A]0 = 0.664m and t1/2 = 103s, we get:\[\frac{103}{2}= \frac{1}{k} \frac{1}{0.664}\]Multiplying both sides with 0.664k:\[k*\frac{103}{2}*0.664 = 1\]Dividing second equation by first equation:\[\frac{k*\frac{103}{2}*0.664}{k*\frac{103}{2}*0.154}= \frac{0.664}{0.154}\] Simplifying the above equation, we get:\[k=6.74*10^{-5}s^{-1}\]Therefore, the order of reaction is 1 and the value of the rate constant is 6.74*10^-5s^-1.
Learn more about order of the reaction at: brainly.com/question/14957291
#SPJ11
How would this model be like after being separated chemically? Please help!!

Answers
A separation process is a method that divides a mixture or solution of chemical compounds into two or more different product mixtures. It is a scientific approach of separating two or more substances in order to achieve purity.
How are chemical brews divided?
Mixtures can be separated using a variety of techniques. Chromatography on a solid medium necessitates solvent separation. Distillation involves the use of several boiling points. Evaporation removes a liquid from a solution, leaving a solid behind.
To learn more about Separated chemical mixture refer to:
https://brainly.com/question/552187
#SPJ13
What is the formula of barium phosphite?
a. \(\mathrm{ba}_3\left(\mathrm{po}_3\right)_2\)
b. \(\mathrm{ba}_2\left(\mathrm{po}_3\right)_3\)
c. \(\mathrm{ba}_3\left(\mathrm{po}_4\right)_2\)
d. \(\mathrm{ba}_2\left(\mathrm{po}_4\right)_3\)
e. \(\mathrm{bapo}_3\)
f. \(\mathrm{bapo}_4\)
Answers
The formula of barium phosphite is Ba₃(PO₄)₂.
A salt that is classified as inorganic is barium phosphate. The chemical formula for barium phosphate is Ba₃(PO₄)₂. Although barium phosphate is typically thought of as a colorless solid, it does have a fading odor resembling that of acetic vinegar. The barium phosphate structure was identified by the X-ray crystallography experiment as a connection of barium ions with the phosphate, Ba₂⁺ cations connected to a polyphosphate anion. The salts or esters that are created when tetrahedral PO₄ is transformed into a polyphosphate anion (phosphate). The oxygen atom that the structural unit shares is what binds it together. One of the hydrated forms of barium phosphate is barium phosphate dihydrate.
know more about chemical formula here
https://brainly.com/question/29031056#
#SPJ4
Describe a method to show the effect of changing the temperature of the nitric acid on the rate of reaction. (Include measuring the volume of carbon dioxide gas produced)
Answers
The effect of changing the temperature of the nitric acid on the rate of reaction. (Include measuring the volume of carbon dioxide gas produced) is Rate of reaction increases.
When we increase the temperature of the reaction the rate of reaction also increases. With the increase of the temperature of the nitric acid HNO₃, the rate of the reaction also increases as the molecule moves faster. Arrhenius equation given as :'
k = Ae^(Ea / RT)
or, ln k = -Ea /RT + ln A
where,
k = rate constant
A = Arrhenius constant
Ea = activation energy
R = gas constant
T = temperature
Thus, The effect of changing the temperature of the nitric acid on the rate of reaction. (Include measuring the volume of carbon dioxide gas produced) is Rate of reaction increases.
To learn more about Rate of reaction here
https://brainly.com/question/8592296
#SPJ1
Which ancient building technique could enclose the largest volume of space using the least amount of material
Answers
A dome on pendentives is an ancient building technique that could enclose the largest volume of space using the least amount of material. It is used in architecture.
What is a dome?A pendentive is an instrument used in buildings that allow the colocation of a circular and/or elliptical dome.
This circular and/or elliptical dome can be placed over a square/rectangular room.
The use of domes on pendentives is relatively common in certain architecture schools.
Learn more about domes here:
https://brainly.com/question/1059924
treatment of pentanedioic (glutaric) anhydride with ammonia at elevated temperature leads to a compound of molecular formula c5h7no2. what is the structure of this product? [hint: you need to think about the reactivity not only of acid anhydrides but also of amides and carboxylic acids]
Answers
The structure of the product is drawn.
The reaction between pentanedioic anhydride and ammonia at elevated temperature is an example of amidation reaction. The product formed has a molecular formula of C₅H₇NO₂, which suggests that it has five carbon atoms, seven hydrogen atoms, one nitrogen atom, and two oxygen atoms.
The constitutional isomers with the molecular formula C₅H₇NO₂ are,
Pentanamide (also known as valeramide)
2-Aminopentanoic acid (also known as α-aminocaproic acid)
3-Aminopentanoic acid (also known as β-aminocaproic acid)
Of these three isomers, only 2-aminopentanoic acid and 3-aminopentanoic acid have two oxygen atoms. Therefore, one of these two isomers is the product of the reaction.
To distinguish between the two isomers, we need to consider the conditions of the reaction. The reaction was carried out at elevated temperature, which suggests that it is likely to be a thermal reaction. Under thermal conditions, the reaction is expected to favor the formation of the less substituted amide, which in this case is 2-aminopentanoic acid.
To know more about ammonia, here
https://brainly.com/question/15719562
#SPJ4

A solution contains a mixture of cl- and br- ions. can both be positivevly identified?
Answers
Yes, \(Br^{-}\) and \(Cl^{-}\) ions both can be positively identified through precipitation reaction or precipitimetry.
Through titration employing precipitation reaction or precipitimetry, these two ions can both be positively identified. When exposed to Cl- and Br- ions, AgNO3 transforms into silver halides. AgNO3 with Cl- ions precipitates white because AgCl is not particularly soluble in water, whereas AgNO3 with Br- ions precipitates cream.
A very light cream precipitate results from mixing cream and white ppt.
Both halides react as described below:
\(AgNO_{3}+ XCl\)\(= AgCl_{whiteppt.}\)
\(AgNO_{3}+ XBr\) \(= AgBr_{creamppt.}\)
Now, While AgBr does not dissolve in diluted ammonia, this AgCl precipitate does to create an Ag-diammonium ion combination. Two facts, including the fact that the ppt shade is now darker than the prior pale cream, demonstrate this. As a result of the addition of an ammonia solution, it becomes less concentrated, although some cream precipitates persist.
Second, concentrated ammonia dissolves the AgBr precipitate. AgBr precipitates dissolve when cream precipitate is filtered and concentrated ammonia is added. In solution Br- ions are confirmed by this.
\(Ag^{+}+NH_{3}\) ⇄ \((AgNH_{3} )_{2} ^{+}\)
The foregoing reaction switches in the right direction after the addition of diluted ammonia solution, and more and more Ag+ ions are complexed, producing the soluble form of Ag-diammonium complex.
Brown globules are produced when CHCl3 is added to the mixture and agitated.
Learn more about ions here:
https://brainly.com/question/269828
#SPJ4
2. What happens when hydrochloric acid (HCl) is added to the solution? Do the relative concentrations of H+, CH3COOH, or
CH3C00 change when HCl is added to the solution?
Answers
Answer: A molecule of hydrochloric acid, for example, is composed of a hydrogen atom and a chlorine atom. When these molecules dissolve into water, they separate into a positively charged hydrogen ion and a negatively charged chlorine ion. ... Only some of the molecules of weak acids disassociate when added to water.
Explanation:

Explain how a synthesis reaction is useful to a pharmacologist
Answers
In most drug discovery efforts, compound synthesis is regarded as the rate-limiting phase to accommodate various functional groups.
One of the most typical kinds of chemical reactions is a synthesis reaction, also known as a direct combination reaction. A + B AB is the result of the reaction between two and more chemical species in a synthesis.
The synthesis process is simple to identify in this form since there are more reactants then products. One bigger compound is created when multiple reactants come together. Synthesis reactions can be thought of as the opposite of breakdown processes. In most drug discovery efforts, compound synthesis is regarded as the rate-limiting phase to accommodate various functional groups.
To know more about synthesis reaction, here:
https://brainly.com/question/16987748
#SPJ1
