Carbon tetrahydride gas reacts with oxygen gas. What is the skeleton and balanced equation for this problem.
Answers
Answer:
CH4+2O2→CO2+2H2O
Explanation:
CH4+2O2→CO2+2H2O
Related Questions
How is polyethene formed?
A. None of these
B. The double bond on reactive ethene molecules breaks allowing them to bond with each other and form a chain.
C. Double bonds are very strong. This means when ethene is heated, hydrogens will be removed before the double bonds break. When ethene molecules lose hydrogens, their carbons bond with each other forming branching chains.
D. Polyethene is formed by the formation of double bonds between carbons in ethane molecules.
Answers
Answer:
c
Explanation:
because 2× is strong. hydrogen will be remove
Explanation:
Poly(ethene) is formed when several monomers of ethene (an alkene) are joined together. Ethene can take part in this type of reaction as it is has a double bond (it's unsaturated) which opens up to allow multiple monomers to form a chain. This reaction occurs when ethene is heated and put under pressure in the presence of a catalyst.
4. A container with a volume of 25.47 L holds 1.050 mol of oxygen gas (02) whose
molar mass is 31.9988 g/mol. What is the volume if 7.210 g of oxygen gas is
removed from the container, assuming the pressure and temperature remain
constant?
Answers
The initial volume of the container can be calculated using the ideal gas formula, PV = nRT, where P is pressure, V is volume, n is number of moles, R is gas constant, and T is temperature:
What is the Avogadro's law worked example?The best illustration of Avogadro's law is when a balloon is inflated. The volume of the balloon grows as you add moles of gas. Similar to how a balloon loses gas and its volume as you collapse it
What does Class 11 of the Avogadro Law entail?According to Avogadro's law, all gases with an identical volume and the same temperature and pressure have an equal number of molecules. The volume of an ideal gas at a certain mass.
To know more about molar mass visit:-
brainly.com/question/21133305
#SPJ9
What role does the solubility, polarity, melting point, and
molecular mass of an organic sunscreen play in determining how good
the sunscreen is
Answers
The solubility, polarity, melting point, and molecular mass of an organic sunscreen play a crucial role in determining how good the sunscreen is.
Solubility:Solubility determines how well the sunscreen will dissolve in a liquid, particularly water. Water is a universal solvent that may wash away sunscreen, particularly if it is not water-resistant. Thus, the greater the solubility, the less effective the sunscreen will be.
Polarity:UV radiation from the sun might induce photochemical reactions in organic sunscreens, causing them to degrade and become less effective. The polarity of a sunscreen, on the other hand, may prevent this from occurring. This is because the polarity of a sunscreen can impact its capacity to absorb UV radiation. A higher polarity sunscreen will absorb more radiation, lowering the chances of the sunscreen deteriorating.
Melting point:The melting point of a sunscreen is a measure of its ability to resist thermal decomposition. The sun can heat up the skin and the sunscreen, so a sunscreen with a higher melting point is more likely to survive and maintain its effectiveness.
Molecular mass:The molecular mass of a sunscreen determines its thickness. A sunscreen with a lower molecular mass is lighter and more prone to washing off. A sunscreen with a higher molecular mass is denser and will cling to the skin, making it more effective.To summarize, the solubility, polarity, melting point, and molecular mass of an organic sunscreen all play important roles in determining how good the sunscreen is.
Learn more about sunscreen at
https://brainly.com/question/24064008
#SPJ11
How much 8.0 M stock solution is required to prepare 100.0 mL of 2.5 M
solution?
Answers
To prepare a 100.0 mL solution of 2.5 M concentration, we can use the formula we need 31.25 mL of the 8.0 M stock solution to prepare 100.0 mL of a 2.5 M solution.
What do you understand by a solution ?A solution is a homogeneous mixture of two or more substances, in which the components are uniformly distributed throughout the mixture on a molecular level.
A solution is composed of a solute (the substance that is dissolved and a solvent the substance that does the dissolving.
To know more about solution visit :
brainly.com/question/30665317
#SPJ1
True or false; A solution always contains only one solvent.
Answers
A solution is defined as a mixture of two or more substances, usually, a solute and a solvent, and the difference between these two are in quantity, solute represents the smallest amount and solvent will represent the highest amount, and while you can have more than one solute, you can only have one solvent for a solution. Therefore the statement is true
What is the temperature of a gas if the container has a volume of 2,300 mL, with a pressure of 932 mmHg and 3.51 moles?
Answers
Answer:
The preceding temperature is equivalent to approximately 9.6 K => -263.5 °C
Explanation:
Given the provided problem, the formula may be substituted by the corresponding values:
Pressure (P)= 932 mmHg ==> 1.2 atm
Volume (V) = 2,300 mL ==> 2.31 L
Moles (n) = 3.51 moles ==> 3.51 mol
Proportional constant of ideal gas constant: (R) = 0.08206
Therefore, we can equate the following:
T= (PV)
nR
T= (1.2 atm · 2.31 L)
(3.51 mol · 0.08206)
T= 9.6 K ==> -263.5 °C
Thus, the cumulative temperature given the substituents/substance is equivalent to -263.5 °C.
Given a solution with a hydrogen ion concentration of 9.9 x10-13 M, calculate the
pОН. .
Answers
Answer: The pOH value is 2.
Explanation:
Given: \([H^{+}] = 9.9 \times 10^{-13} M\)
pH is defined as the negative logarithm of hydrogen ion concentration.
Hence, pH of the given solution is calculated as follows.
\(pH = -log [H^{+}]\\= - log (9.9 \times 10^{-13})\\= 12\)
The relation between pH and pOH is as follows.
pH + pOH = 14
Therefore, pOH is calculated as follows.
pH + pOH = 14
12 + pOH = 14
pOH = 14 - 12
= 2
Thus, we can conclude that the pOH value is 2.
The dog will eat more food to store fat and grow more hair.
The dog will lay in the sunlight to transpire
The dog will pant to circulate air throughout the body to cool
down
3.
The dog will grow a thick winter coat to keep warm during the
In the summer months, what is this dog's likely response to the rising temperatures?
summer
CLEAR ALL
Answers
hibernation exclamation mark
Explain why the kidneys are important to our survival
1. They filter waste from the blood
2. They remove urine from the body
3. They increase the sugar level in the blood
4. They make new blood for the body
Answers
The kidneys are important to our survival because they play a critical role in maintaining the balance of fluids and electrolytes in the body, as well as removing waste and toxins from the blood. Options 1 and 2 are correct.
The kidneys filter waste from the blood, such as excess water, salts, urea, and other waste products. They remove urine from the body, which helps to maintain the proper balance of fluids and electrolytes in the body. The kidneys do not increase the sugar level in the blood, but they do play a role in regulating blood sugar levels by producing hormones such as renin and erythropoietin.
The kidneys do not make new blood for the body, but they do produce a hormone called erythropoietin that stimulates the production of red blood cells in the bone marrow. The kidneys are essential to our survival because they help maintain the proper balance of fluids and electrolytes, remove waste and toxins from the blood, and play a role in regulating blood pressure and red blood cell production. Options 1 and 2 are correct.
To know more about the Kidneys, here
https://brainly.com/question/11008782
#SPJ4
Hi!, please help :)
Answers
Answer:
5.8
Explanation:
a brief description of atoms and how they relate to
molecules and compounds
Answers
Answer:
Explanation:
Atoms are the thing that make up molecules and compounds. Molecules contain two or more atoms and are held together by covalent bonds
What is the density of a book with a mass of 60g and a volume of 20 cm3? *
Answers
Answer:
\(3 g/cm^{3}\)
Explanation:
Density is a measure of a substance's mass over its volume.
d = m/v
Therefore d = 60g/20cm3 = 3 g/cm3
A container holds three gases at a total pressure of 800 kPa. If the partial pressure of the first gas is 100 kPa and the partial pressure of the second gas is 300 kPa, what is the partial pressure of the third gas?
Answers
We can make use of the fact that the total pressure of the mixture is the same as the sum of the partial pressure of all the gases in the container. As a result, 400 kPa is the third gas' partial pressure.
What exactly is a partial pressure law?The overall pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each of the constituent gases, according to Dalton's law of partial pressures. The partial pressure is the pressure that each gas would have if it occupied the same volume of the mixture at the same temperature on its own.
Mathematically, this can be stated as:
P_total = P_1 + P_2 + P_3
When we plug these figures into the formula, we get:
800 kPa = 100 kPa + 300 kPa + P_3
When we simplify this equation, we obtain:
800 kPa = 400 kPa + P_3
By taking away 400 kPa from both sides, we arrive at:
P_3 = 400 kPa
To know more about partial pressures visit:-
https://brainly.com/question/15075781
#SPJ1
83.20 grams of manganese to moles
Answers
To find the moles present in 83.20 grams of manganese we must use the molar mass of manganese. This mass is equal to 54.94g/mol. So the moles of manganese (Mn) will be:
\(molMn=givengMn\times\frac{1molMn}{MolarMass,gMn}\)\(molMn=83.20gMn\times\frac{1molMn}{54.94gMn}=1.51molMn\)Answer: 83.20 grams of manganese are equivalent to 1.51 moles
Indicate the charge the following elements as they achieve the noble gas configuration.
Ga O Br P Rb As
S Mg Al Se Li I
Answers
Answer:
See explanation
Explanation:
Ga is in group 13 hence it must loose three electrons to form Ga^3+ in order to achieve the noble gas configuration because it has three electrons on its outermost shell.
O is in group 16 hence it must accept two electrons in order to attain the noble gas configuration to form O^2- since oxygen has six electrons on its outermost shell.
Br in group 17 has seven electrons in its outermost shell hence it must form Br^- (gain one electron) in order to attain the noble gas configuration.
P in group 15 must accept three electrons and form P^3- in order to attain the noble gas configuration since it has five electrons on its outermost shell.
S is in group 16 hence it must accept two electrons in order to attain the noble gas configuration to form S^2- since sulphur has six electrons on its outermost shell.
Mg in group 2 has two electrons on its outermost shell and must loose both to attain the noble gas configuration forming Mg^2+.
Al is in group 13 hence it must loose three electrons to form Al^3+ in order to achieve the noble gas configuration because it has three electrons on its outermost shell.
Se is in group 16 hence it must accept two electrons in order to attain the noble gas configuration to form Se^2- since selenium has six electrons on its outermost shell.
Lithium is in group 1 and must loose its only outermost electron in order to attain the noble gas configuration to form Li^+.
Rb is in group 1 and must loose its only outermost electron in order to attain the noble gas configuration to form Rb^+.
As in group 15 must accept three electrons and form As^3- in order to attain the noble gas configuration since it has five electrons on its outermost shell.
I in group 17 has seven electrons in its outermost shell hence it must form I^- (gain one electron) in order to attain the noble gas configuration.
In the geocentric theory the earth is the center of the universe.
A. True B. False
Answers
I am not quite sure but I would say A. true. Althought I am not 100% shure this is right.
But here is the reason that I chose this because in a geocentric model the sun, moon, stars, and plantes all orbit around the earth.
Hope this helps :))
Answer: the answer is a which is true .
Explanation: the sun, moon, stars, and plantes all orbit around the earth.geocentric model, any theory of the structure of the solar system (or the universe) in which Earth is assumed to be at the centre of it all, so it is correct . HHOPE IT HELPS YOU .PLEASE GIVE BRAINLIEST .THANKS .
What is the two major types of chemical bonding?
Answers
Answer:
Ionic bond and covalent bond
Explanation:
The two main types of chemical bonding are ionic bond and covalent bond.
In an ionic bond, there is a transfer of electrons from one atom to another. Usually from a metal to a nonmetal leading to the existence of an ion pair. Ionic compounds are soluble in water and have high melting and boiling points due to the strong electrostatic interaction between ions in the compound.
A covalent bond involves sharing of electrons between atoms. In an ordinary covalent bond, the shared electrons are furnished by the two bonding atoms while in a dative covalent bond, the two bonding electrons are furnished by only one of the bonding species.
hich option is an ionic compound?
Responses
NO2
upper case N O subscript 2 end subscript
SO3
upper case S O subscript 3 end subscript
CO
upper case C O
LiCl
Answers
NO₂ , SO₃ and CO are covalent compounds and LiCl is ionic in nature.
What are differences between covalent and ionic compounds?The definition of an ionic compound is chemical compound composed of ions which is held together by electrostatic forces i.e. held together by ionic bonds. They are formed by ions of opposite charge. The compound is neutral but it consists of a positively and negatively charged cations and anions.
Ionic bonds transfer electrons, covalent bonds share them more easily .Ionic compounds tend to have higher melting points and boiling points while covalent compounds have lower melting & boiling pointsIonic compounds have more polar molecules and covalent compounds lessOrganic compounds tend to have covalent bondsIonic compounds are usually between metal and a non-metal. Non-metal with non-metal compounds are covalent.Ionic compounds have ions in solution or in molten state and conduct electricityIonic bonds are stronger than covalent bondsIonic compounds tend to be a solid with definite shape at room temperature while covalent compounds are usually gases, liquids or soft solidsIonic compounds often do not dissolve in organic solvents while covalent compounds do.Learn more about ionic compounds at https://brainly.com/question/2687188
#SPJ10
Balance the following chemical equation.
CO2(g)+CaSiO3(s)+H2O(l) --> SiO2(s)+Ca(HCO3)2(aq)
Answers
Balanced Equation: CaSiO3 + 2 CO2 + H2O = Ca(HCO3)2 + SiO2
One or more chemical substances can react with each other and form one or more new substances through a process which is referred to as chemical reaction.
Reactants are the elements required for a reaction's initialization.
The components that remain after a reaction are called products.
Each kind of atom is to be present on both the reactant and product side of a chemical equation in a chemical reaction in order for it to be balanced.
On both sides of balanced chemical equations, the same number and type of each atom can be found.
A balanced chemical equation must have coefficients that are the smallest whole number ratio.
Mass is always conserved in chemical processes.
To find more Chemical reaction balance here:
https://brainly.com/question/29130807#
#SPJ4
Determine the molecular formula of a compound whose molecular mass is 60.00 g/mol and has an empirical formula of ch4n. ch4n c2h5n2 c2h8n2 c3h12n3
Answers
Molecular formula of a compound whose molecular mass is 60.00 g/mol and has an empirical formula of C₂H₈N₂.
We know the empirical formula and thus the molar mass of the empirical formula, we simply need to find out how many of these fit into the molar mass of the molecular formula.
In this problem, we have an empirical formula of CH₄N
so the molar mass is 12 + 4 + 14 = 30 g/mol.
Molecular formula mass/Empirical formula mass=60 g/mol/30 g/mol=2
The molecular formula is TWICE that of the empirical formula.
Molecular formula = 2XCH₄N = C₂H₈N₂
Learn more about the Molecular formula with the help of the given link:
https://brainly.com/question/14425592
#SPJ4
Answer:
C2H8N2
Explanation:
edge 2023
which of your body structures was the effector in the reaction time test? what was your motor response?
Answers
Skeletal muscle was the Effector in the reaction time test and motor response reflects the muscular component of reaction time.
The Effector in the reaction time test was the skeletal muscle in the finger which is used to press the button. muscle and glands produces a specific response to a stimuli's and the motor response was reaction time test is the time between electromyographic activity and movement and the motor response is the response which reflects the skeletal muscle component of reaction time.
learn more about Effector
brainly.com/question/3190796
#SPJ4
ercent Yield For the following balanced chemical reaction: 2 Hg O2 -- 2 HgO If you start with 33 grams of Hg and you produce an actual amount of 25 grams HgO in the lab what is the percent yield
Answers
The Percent Yield for the following balanced chemical reaction 75.76%.
What exactly is percent yield?Percent yield in chemistry is the percentage of the product's weight to its theoretical yield. In order to quantify the outcome in percent, we divide the experimental yield by the theoretical yield and multiply the result by 100.
Why do we compute percent yield?Because many chemical reactions produce byproducts, not all of the reactants in the equation actually undergo reaction, percent yield is significant. A poor percent yield in product manufacturing would mean that the corporation is wasting resources, including money and reactants.
To know more about Percent Yield visit-
https://brainly.com/question/17042787
#SPJ4
6. In which of the following will the bulb glow?
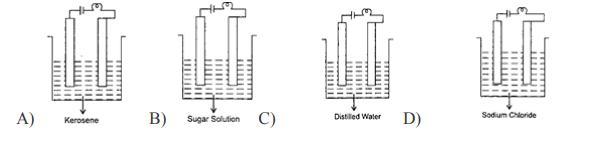
Answers
Answer:
Kerosene
Explanation:
You use process of elimination in this question
None of them except for Kerosene can power a bulb
Explanation:
sodium chloride
thank me later
which is a weak electrolyte? question 15 options: table salt (sodium chloride) sugar corn oil vinegar (acetic acid)
Answers
Answer:
The weak electrolyte is Aqueous acetic acid.
Which term describes the conversion of substances into different substances? (1 point)
O ionic bonding
O fossilization
O chemical reaction
O photosynthesis
Answers
Chemical reaction is the term which describes the conversion of substances into different substances.
What is chemical reaction?Chemical reactions are defined as reactions which occur when a substance combines with another substance to form a new substance.Alternatively, when a substance breaks down or decomposes to give new substances it is also considered to be a chemical reaction.
There are several characteristics of chemical reactions like change in color, change in state , change in odor and change in composition . During chemical reaction there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
There are three types of chemical reactions:
1) inorganic reactions
2)organic reactions
3) biochemical reactions
Learn more about chemical reactions,here:
https://brainly.com/question/14929452
#SPJ1
what is the mass of an atom if the mass number is 101?
Answers
Is Sucrose a ionic or covalent bond
Answers
Answer:
covalent bond
Explanation:
hope this helps :D
Eukaryotic cells contain a structure called the _______ which is distinct central organelle that contains the cells ____ material.
Answers
Eukaryotic cells are organelles with a developed nucleus. Eukaryotic cell contains a structure called cytoskeletal structure which is distinct central organelle that contains the cells genetic material DNA.
What is Eukaryotic cells?There are two type of cells based on the nucleus. If the cell is having a well developed nucleus it is called eukaryotic cells and the cell which have no developed nucleus is called prokaryotic cell.
Eukaryotic cells contains nucleus, cytoplasm, endoplasmic reticulum, lysosomes, mitochondria and ribosomes.
In eukaryotic cells contains a cytoskeletal structure inside cytoplasm consist of fibers, microtubules and filaments which make it strong.
The genetic material DNA is placed inside this cytoskeleton. Cytoskeleton provide perfect shape to the cell and helps to stimulate the cell movement.
Therefore, the special organelle contained in eukaryotic cells which contains the genetic material DNA.
To learn more about eukaryotic cells, refer the link below:
https://brainly.com/question/11351358
#SPJ1
A teacher gives students four liquids commonly found in the kitchen - vinegar, apple juice, dish detergent, and milk -
and pH indicator strips, which measure acidity. The teacher asks the students to put the liquids in order from most
acidic to most basic.
What type of investigation could the students conduct to determine the correct order for the liquids? |
The type of liquid is the
The pH is the
Answers
Answer:
I think pH strips wouldn't be enough to arrange them in the recquired order what u need is a universal indicator
Explanation:
With the use of universal indicator u can be able to arrange them
Answer:
Comparative, independent, dependent
Explanation:
You're comparing which has the most ph, and the ph is dependent on the liquid leaving the liquids to be independent.
How many grams of AgCl are produced from 10.0 g AgNO3?
Answers
Answer:
Equation of Reaction = AgNO3 (aq) + NaCl (aq) → AgCl (s) + NaNO3 (aq)
Steps:
1. Find the moles of AgNO3 with formula n= Mass/Mr (molecular mass)
so you will get moles= 10.0/ 169.87 = 0.05886854654
2. Using stoichiometry you know the equation has 1 mole so the moles of AgCl are gonna be the same; therefore, you will use again the same formula.
3. moles= mass/molecular mass
0.05886854654 = x/ 143.32
You rearrange the formula to find the mass
so mass = moles x molecular mass
mass = 0.05886854654 x 143.32
mass= 8.44 grams
Explanation: