Centripetal acceleration is in a
Answers
Related Questions
Mike is performing an experiment in a laboratory. in the experiment, he wants to prevent heat from escaping from a hot iron rod. he hangs the rod from a thread and covers it with a thick plastic sheet to prevent air from flowing around it. the plastic sheet surrounds the rod but does not touch it. after some time, mike finds that the iron rod has cooled down considerably.why did the iron rod cool down? a. heat left the iron rod in the form of waves traveling through space. b. the plastic sheet increased the surface area of the iron rod. c. atoms in the iron rod transferred energy directly to atoms in the plastic sheet. d. energy traveled into the rod through the thread.
Answers
The correct answer is: a. Heat left the iron rod in the form of waves traveling through space.
The cooling of the iron rod in this scenario is primarily due to heat transfer by radiation. When an object, such as the hot iron rod, has a higher temperature than its surroundings, it radiates heat energy in the form of electromagnetic waves, including infrared radiation. These waves can travel through space without the need for a medium. By covering the iron rod with a plastic sheet, although it prevents airflow and reduces convective heat transfer, does not significantly affect the radiation of heat energy from the rod. The radiation can still occur as the waves can pass through the plastic sheet and escape into the surroundings.
Therefore, the cooling of the iron rod is primarily attributed to heat leaving the rod in the form of waves traveling through space, as mentioned in option A.
Learn more about iron rod here:
https://brainly.com/question/31924783
#SPJ11
the pH of a solution is 2.0. what is the [OH^-] concentration?
Answers
What is the minimum hot holding temperature for fried chicken?.
Answers
Answer:
130
Explanation:
Maintain hot food at 135°F or above. Properly cooked roasts may be held at 130°F or above.
135°F is the minimum hot holding temperature for fried chicken. The most often used scales are indeed the Celsius scale.
What is temperature?Temperature is a physiological measure that quantifies our feelings of hotness and coolness. A thermometer is used to measure temperature.
Thermometers were calibrated in a variety of temperature scales that have traditionally been defined by various reference points including thermometric substances.
The most often used scales are indeed the Celsius scale, with the unit sign °C, the Fahrenheit level (°F), as well as the Scale parameter (K), with the latter being mostly used for scientific reasons. The kelvin is one of the International System of Units' seven basic units (SI). 135°F is the minimum hot holding temperature for fried chicken.
Therefore, 135°F is the minimum hot holding temperature for fried chicken.
To learn more about temperature, here:
https://brainly.com/question/23411503
#SPJ2
True or False?
When it is summer in the Northern Hemisphere, it is summer all around the word
Answers
give the nuclear symbol, including superscript and subscript, for each type of radiation.
alpha particle: He beta particle: gamma ray: Y Which type of radiation has a negative charge? a. alpha b. beta
c. gamma Which type of natural radiation is pure energy? a. gamma b. alpha
c. beta
Which type of natural radiation has the most massive particles? a. beta b. alpha c. gamma
Answers
The subscript in alpha radiation stands for the charge on the nucleus (i.e., the quantity of protons or atomic number, also known as the Z number), and the superscript is the mass number.
In beta radiation, the particle's charge is indicated by the subscript -1, and its almost completely absent mass is indicated by the superscript 0. (no protons or neutrons). The radioactive thorium-234 nucleus is another illustration.
Alpha, beta, and gamma are what?While alpha and beta particles have positive and negative charges, respectively, gamma rays are neutral. An alpha particle is created when two protons and two neutrons come together. Beta particles are high-energy electrons. Photons, which are electromagnetic energy waves, are gamma rays.
To know more about alpha radiation visit:-
https://brainly.com/question/6070167
#SPJ4
Why do reactions need to be balanced?
A. Because the reactants must be the exact same as the products
B. Because of the conservation of energy
C. Because the volume of reactants must equal the volume of
products
D. Because atoms are not lost or gained in a chemical reaction
Answers
Answer:
A part.
Explanation:
Because the reactants must be the exact same as the the products.
Which of the following is the best example of solar energy being converted into chemical energy?
photosynthesis
heating of the road
formation of clouds
evaporation of water
Answers
Answer:
It's photosynthesis.
what mass of ZnCI2 can be prepared from the reaction of 3.27 grams of zinc with 3.30 grams of HCI?
Zn + 2HCI —-> ZnCI2 + H2
Answers
Answer:
Zn. + 2 HCl ----------> ZnCl2. + H2
Explanation:
Mass of Zn in mole = 3.27/ 65.38= 0.050010.... mol
Mass of HCl in mole = 3.3/ 73= 0.044....mol
Hence, limiting reagent is HCl
Molar mass of ZnCl2= 136.38g
Let mass of ZnCl2 be x
x = 3.3*136.38÷ 73 = 6.165...
So, the mass of ZnCl2 is 6.17 g
1. How many grams of iron (II) oxide can be produced from 3.4 g of iron in this balanced equation?
4Fe +3022Fe₂O3
2. How many grams of magnesium carbonate is produced from 6.7 g of magnesium oxide in this
balanced equation?
MgO + CO₂ → MgCO3
3. How many grams of calcium hydroxide is produced from 9.4 g of calcium chloride in this balanced
equation?
CaCl₂ + 2NaOH → 2NaCl + Ca(OH)2

Answers
Answer:
3.12g FeO3
14.33g MgCO3
5.92g Ca(OH)2
Explanation:
For the first problem, convert grams of Fe to mols of Fe.
molar mass of Fe: 55.845
Grams of Fe: 3.4g
3.4/55.845 = 0.06 mol Fe
There are 4 Fe for every 2 FeO3
So.. 0.06(2/4) = 0.03 mol FeO3
Multiply the mols by the molar mass of FeO3
Molar mass of FeO3: 103.845
0.03 * 103.845 = 3.12 g FeO3
the gram staining procedure is best described as a(n) __ staining technique.
Answers
Answer:
differential
Explanation:
According to openstax.org, "Gram-staining is a differential staining technique that uses a primary stain and a secondary counterstain to distinguish between gram-positive and gram-negative bacteria."
i need help yall ill mark brainliest :)

Answers
Answer:
How does non-human life in an urban ecosystem differ from that in an undeveloped forest ecosystem?
Explanation:
In forest eco system the people are uncivilized and not so much educated. And in urban eco system people are intelligent as well as educated this is the main reason. In an urban ecosystem, the only non-human life that live there are the ones that are able to survive under the conditions. However, in undeveloped forest ecosystems, there’s barely any pollution. Therefore, a bigger variety of non-human life is able to live here.
Mark brainliest if you can please :)
how many molecules of HI are needed to produce 72.54g of BaL2? Equation: BaSO4+HI=Bal2+H2SO4
Answers
The number of molecules of HI that are needed to produce 72.54g of BaL2 is6.365 x 10^23.
How to find the molecules needed?To determine the number of molecules of HI needed to produce 72.54g of BaL2, we need to first find the moles of BaL2 and then use the balanced chemical equation to find the moles of HI.
First, we'll find the moles of BaL2:
72.54 g of BaL2 / 137.3 g/mol = 0.529 moles of BaL2
Next, we'll use the balanced chemical equation to find the moles of HI:
BaSO4 + 2HI -> BaL2 + H2SO4
From this equation, we can see that for every 2 moles of HI that react, 1 mole of BaL2 is produced. Therefore, to produce 0.529 moles of BaL2, we need 0.529 x 2 = 1.058 moles of HI.
Finally, we'll convert the moles of HI to the number of molecules:
1.058 moles of HI x 6.022 x 10^23 molecules/mole = 6.365 x 10^23 molecules of HI.
Therefore the number of molecules of HI needed to produce 72.54g of BaL2 is approximately 6.365 x 10^23.
Learn more about molecules here:https://brainly.com/question/26044300
#SPJ1
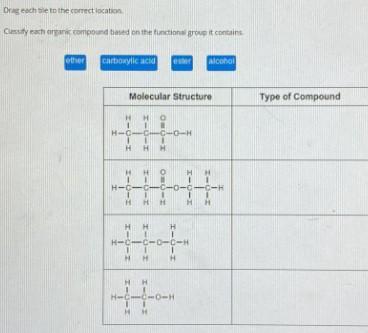
Drag each tile to the correct location.
Classify each organic compound based on the functional group it contains,
ether
carboxylic acid
ester
alcohol
Answers
answer: Carboxylic acid, Ester, Ether and Alcohol
Explanation: Carboxylic acid is the one which has COOH group which includes carbonyl group and OH.
And ester is a derivative of Carboxylic acid which has COOR . It has -OR along with Carbonyl group.
And ether has C-O-C skeleton
And alcohol has R-OH as a general formula.er:
Explanation:
The given compounds can be classified based on the functional groups they contains. The first compound is an acid containing the COOH group. Second one is an ester with the COOR group, third one is as an ether and the last compound in the column is an alcohol.
What are organic compounds?Organic compounds are compounds containing carbon-carbon backbone with hydrogen C-H bonds also. There are different types of organic compounds namely, acids, alcohols, esters, ketones, aldehydes etc.
These organic compounds are classified based on the functional groups present in them. The compounds containing OH groups are called alcohols whereas those containing COOH groups are carboxylic acids. Those with OR group are ethers and compounds with COOR group that is COO in between two carbon chains are esters.
From the given column, the first compound is carboxylic acid namely propanoic acid. Second one is an ester called ethyl propanoate and third one is an ether namely methyl ethyl ether and the last one is an alcohol namely ethanol.
To find more on organic compounds, refer here:
https://brainly.com/question/5994723
#SPJ5
Your question is incomplete. But your complete question probably was as uploaded in the image.
Food waste, like a feather or a bone, fall into food, causing
contamination.
Physical
Chemical
Pest
Cross-conta
Answers
Answer:
what do I need to do?..
..
Feather or bone when fall into food causing Physical contamination.
Food waste, like a feather or a bone etc fall into food causing Physical contamination because the contaminants such as feather and bone are mixed in the food physically not chemically. These contaminants can be removed through physical means such as with the help of hand.
Some contaminants mix with the food chemically means they react with the food and produce something new. Such type of contaminants are present in liquid form so we can conclude that feather or a bone etc when fall into food causing Physical contamination.
Learn more about contamination here: https://brainly.com/question/3459685
Learn more: https://brainly.com/question/25889410
a 110.-g sample of copper (specific heat capacity 5 0.20 j/8c ? g) is heated to 82.48c and then placed in a container of water at 22.38c. the final temperature of the water and copper is 24.98c. what is the mass of the water in the container, assuming that all the heat lost by the copper is gained by the wate
Answers
The mass of water is 231.71kg
What is specific heat capacity?The specific heat capacity of a substance is the quantity of heat required to increase the temperature of a unit mass of substance by 1°C or 1k. It is measured in J/kg/K. It is denoted by c.
The quantity of heat required is expressed as H= mc∆k. where m is the mass and k is temperature.
From the law of calorimetry, heat lost = heat gained. The specific heat capacity of water and copper are 400J/kg/k and 4200J/kg/k
heat lost by copper = 110×400× ( 82.48-24.98)
= 44000× 57.5 = 2530000J
heat gained by water = m× 4200 × (24.98-22.38)
= 4200× 2.6×M = 10920m
therefore 2530000= 10920m
where m is the mass of water
m= 2530000/10920
m= 231.71kg
therefore the mass of the water is 231.71kg
learn more about specific heat capacity from
https://brainly.com/question/28825214
#SPJ1
What effect does the concentration of reactants have on the rate of a
reaction?
A. Changing the concentration has no effect on the reaction rate.
B. Increasing the concentration decreases the rate of the reaction.
O C. Increasing the concentration increases the rate of the reaction.
O D. The effect of concentration on rate varies within the reaction.
Answers
Answer:
C
Explanation:
The answer is C as concentration is a major factor affecting the rate of reaction and it has a direct relationship with the rate of reaction.
The effect that the concentration of reactants has on the rate of a reaction is increasing the concentration increases the rate of the reaction. The correct option is C.
What is a reactant?A reactant is a part of a reaction that is the first part of the reaction. Reactant can be more than one in the reaction. The different reactant reacts, and form a totally different product.
H2 + O2 = H2O
The hydrogen and oxygen are the reactants of the reaction.
Thus, the correct option is C. Increasing the concentration increases the rate of the reaction, as concentration plays a significant role in determining the rate of reaction and is directly related to it.
Learn more about reactants, here:
https://brainly.com/question/14449229
#SPJ5
can you hellp me plez

Answers
Answer:
srry, I don't understand, I would need more info
31.1 grams of O2 and 84.3 grams of F2 are placed in a container with a volume of94.9 L. Find the total pressure if the gasses are at a temperature of 55.77 ° c
Answers
In this question, we have:
31.1 grams of O2
84.3 grams of F2
94.9 L of total volume
55.77°C of temperature which is equal to 328.92 K
Now, to find the pressure of this container, we can find the number of moles of each gas, and add both values together making it one value of moles and then we will use the Ideal gas law to find the pressure, so let's start with O2
The molar mass of O2 is 32g/mol and we have 31.1 grams
32g = 1 mol
31.1g = x moles
x = 0.972 moles of O2
Now for F2, the molar mass is 38g/mol, and we have 84.3 grams
38g = 1 mol
84.3g = x moles
x = 2.22 moles of F2
Now we add these values, 0.972 + 2.22 = 3.192 moles
And now we can use the ideal gas law formula:
PV = nRT
Remember that R is the gas constant, 0.082
P * 94.9 L = 3.192 * 0.082 * 328.92
94.9P = 86.1
P = 0.91 atm
What happens to atomic radius on going from left to right in a period in a periodic table?
A. Remains constant
B. Decreases first and then remains constant
C. Decreases
D. Increases
Answers
Please give brainliest I need two more!!!!!!!!!!!!!!!
Image transcription text(a) diameter of beaker [52mg (b) mass of sugar (0) volume of alcohol (d) temperature of air ... Show more
Answers
(a) diameter of beaker: Vernier caliper
(b) mass of sugar: weighing balance
(c) volume of alcohol: volumetric flask
(d) temperature of air: Thermometer
Understanding Laboratory InstrumentA laboratory instrument is a tool used in scientific research, experiments, or analysis to measure, monitor, or analyze various parameters, properties, or quantities. These instruments are specifically designed to provide accurate and precise measurements in a controlled laboratory environment.
Here are explanations of the laboratory instruments mentioned:
1. Vernier caliper: A Vernier caliper is a measuring instrument used to measure dimensions such as length, diameter, or thickness with high precision. It consists of an outer scale and a sliding inner scale, allowing for precise measurements. The caliper can be used to measure the diameter of a beaker by gently placing the beaker between the jaws of the caliper and reading the measurement from the scales.
2. Weighing balance: A weighing balance, often referred to as a scale, is used to measure the mass of an object or substance. It provides an accurate measurement of weight by balancing the object being measured against known weights. To measure the mass of sugar, you would place the sugar on the weighing pan or platform of the balance and read the displayed mass.
3. Volumetric flask: A volumetric flask is a glass container used to measure and hold a specific volume of liquid. It has a precise volume calibration mark etched on its neck, indicating the intended volume. To measure the volume of alcohol, you would use a volumetric flask of the appropriate size, add the alcohol to the flask up to the calibration mark, and ensure that the meniscus of the liquid aligns precisely with the mark.
4. Thermometer: A thermometer is an instrument used to measure temperature. It typically consists of a glass tube with a calibrated scale and a temperature-sensing element, such as mercury or a digital sensor. To measure the temperature of the air, you would place the thermometer in the air and allow it to equilibrate, then read the temperature indicated on the scale or display.
[Original question attached]
Learn more about laboratory instrument here:
https://brainly.com/question/30395634
#SPJ4

M
(2.1)
2. How many moles is 2.55 x 1026 atoms of Neon?
3
Vou bout 20 r.
Answers
Answer:
423.44 moles
Explanation:
We need to find no of moles in \(2.55\times 10^{26}\ \text{atoms}\) of Neon.
We know that,
\(1\ \text{mol of atoms}=6.022\times 10^{23}\ \text{atoms}\)
\(2.55\times 10^{26}\ \text{atoms}=2.55\times 10^{26}\ \text{atoms}\times \dfrac{1\ \text{mol}}{6.022\times 10^{23}\ \text{atoms}}\\\\=423.44\ \text{mol}\)
It means there are 423.44 moles in \(2.55\times 10^{26}\ \text{atoms}\) of Neon.
Implementing a new system into an organization module by module is called a ____________.
Answers
Implementing a new system into an organization module by module is called a Phased Approach.
The "phased approach," as the name implies, is a project planning strategy in which anything new, such as a software solution, is introduced in stages rather than all at once.
Rather than installing and rolling out new software across an entire organization at the same time, older systems/methodologies are gradually replaced. Phased implementation necessitates extensive project planning to determine which software functions should go live first and for which departments.
This process allows you to keep the most critical departments operational while others transition to the new system. However, phasing may not be necessary for every company or project. As software and technology become more complex, however, the phased approach reduces risk and often makes the most business sense.
Find more on phase related questions at : brainly.com/question/28162704
#SPJ4
A system conducts 50. J heat to the surroundings while delivering 20. J of work. What is the change in internal energy
Answers
Why are noble gases called "safe" gases?
Answers
Answer:
Noble gases are called "safe" gases because they are inert.
General Formulas and Concepts:
Reading a Periodic TableChemical Properties of Noble GasesExplanation:
Because Noble Gases have filled their outer shell electrons fully, they tend not to form compounds with other elements or react with others to produce another compound.
Take Ne (neon) for instance. It's electron configuration is 1s²2s²2p⁶. The outer most shell is the 2p sublevel, and according quantum numbers, the 2p sublevel only has 3 spaces for 2 electrons each that it can hold. Therefore, Ne has a full outer shell and no electron spots to create a bond with other elements.
How many Molecules of oxygen are produced by the reaction of 7.89 grams of KCIO3
KCIO3 — KCI + O2
Answers
Answer:
5.81×10²² molecules of oxygen.
Explanation:
This a reaction of decomposition:
2KClO₃ → 2KCl + 3O₂
2 moles of potassium chlorate can produce 2 moles of potassium chloride and 3 moles of oxygen.
We determine the amount of salt.
7.89 g. 1mol /122.55g = 0.0644 moles.
If 2 moles of salt, can produce 3 moles of oxygen.
0.0644 moles of salt may produce (0.0644 . 3) /2 = 0.0966 moles.
Let's count the molecules.
1 mol oxygen contains 6.02×10²³ molecules
Then, 0.0966 moles may contain (0.0966 . NA) = 5.81×10²² molecules
chemical change grade 10 mind map
Answers
Chemical change: A chemical reaction is the change of one chemical substance into another chemical substance. For instance: The rusting of iron, the curdling of milk, the digestion of food, breathing, etc.
What is a chemical reaction?
A chemical reaction results in a chemical change because a new material has entirely different properties from the original substance. In a chemical reaction, atoms rearrange themselves.Reactants are the chemicals that participate in a chemical reaction.Products are the new compounds created as a result of a chemical process. An illustration of a chemical reaction is burning magnesium in the air to produce magnesium oxide.2Mg(s) + O2(g) △→ 2MgO(s)The magnesium ribbon is cleaned with sandpaper before being burned in the air. This cleans the magnesium ribbon's surface of the basic magnesium carbonate protecting coating.Reactant: Materials that participate in a chemical reaction are referred to as reactants. Mg and O2, as an example.A product is a newly created substance that results from a chemical reaction. Example: MgO.A chemical reaction is the change of one chemical substance into another chemical substance.
To learn more about chemical reactions, refer to:
https://brainly.com/question/1222323
#SPJ9

Chemical change is the change chemical substance is transformed into another chemical substance.It is irreversible in nature , for example Reaction of medicine in body , milk to curd etc.
What is the difference between chemical and Physical change?1)Physical change temporary or reversible in nature but chemical change is irreversible in nature
2)In physical change there no new product is formed but in chemical change there formation of new product takes .
3) Physical change is change sin shape ,size or state for example freezing of water , melting of wax , and example of Chemical change are Burning of coal, digestion of food
to learn more about chemical change click here https://brainly.com/question/28089135
#SPJ9
La manifestación de un huracán, el movimiento de los aerogeneradores cuando el viento alcanza una velocidad de 3 a 4 metros por segundo; la quema de madera para hacer hervir el agua contenida en un recipiente. Son ejemplos de. (2 ptos.)
Answers
Answer:
Ambos son ejemplos de procesos de transformación de la energía.
El ejemplo del aerogenerador transforma la energía cinética traslacional del viento en energía cinética rotacional del aerogenerador y pérdidas debido a la fricción, la cual es en forma de calor.
En cambio, el ejemplo del hervido del agua es el ingreso de calor por la combustión de madera para aumentar su energía interna.
Explanation:
Son ejemplos de procesos de transformación de energía, por el Principio de Conservación de la Energía se sabe que la energía total de un sistema no se crea ni se destruye, solo se conserva.
El ejemplo del aerogenerador transforma la energía cinética traslacional del viento en energía cinética rotacional del aerogenerador y pérdidas debido a la fricción, la cual es en forma de calor.
En cambio, el ejemplo del hervido del agua es el ingreso de calor por la combustión de madera para aumentar su energía interna.
What are two methods used to help identify the species of insect from the eggs on
human remains? Describe the methods.
Answers
Answer:
The two different methods to help identify the species of insects from the eggs on human remains are by using a small paint brush dipped in water and the other way is by using forceps.Explanation:
mark as brainliestThe insects have been defined as the most successful arthropods. There are far more species in the class Insecta than in any other group of animals. The insects are the only invertebrates with wings.
What are insects?The insects play a very important role in the web of the life in every environment. Their jobs include pollinating flowering plants, since it is the source of food for insectivorous animals and it assist in the decomposition of plants and animals.
The two different methods which help to identify the species of the insects from the eggs on human remains are by using a small paint brush dipped in water and the other way is by using the forceps.
The study of insects and their life cycle are found to be important for the forensic entomologist. This is because it could set a timeline on when the crime is happened. The life cycle of a blowfly starts as an egg and then goes to be maggots.
To know more about insects, visit;
https://brainly.com/question/13277346
#SPJ2
Here is Beck's address:
12 Riverdale Lane, Apartment A
San Jose, California
USA
Imagine each of the five parts of Beck's address represents Earth, the Milky Way, the moon, the solar system, or the universe, based on their sizes. Which of the
following parts of Beck's address would represent the solar system?
O California
O 12 Riverdale Lane
San Jose
O USA
Answers
Answer:
The answer is San Jose
Considering the stereochemistry of the inteediate I below, which of the products would you expect. Explain your answer.
Answers
The expected product is (R)-2-bromobutane.
Stereochemistry plays a crucial role in determining the outcome of chemical reactions. In the given question, the stereochemistry of the intermediate I needs to be considered to determine the expected product.
The intermediate I indicates a chiral carbon center, denoted by an asterisk (*), which means it has four different substituents attached to it. This chiral carbon results in two possible stereoisomers: (R)-2-bromobutane and (S)-2-bromobutane.
When a reaction occurs at a chiral carbon, the stereochemistry of the reactant is usually retained in the product, assuming no racemization or inversion takes place during the reaction. In this case, the intermediate I has an (R) configuration, which implies that the product will also have an (R) configuration.
Therefore, the expected product is (R)-2-bromobutane.
Learn more about (R)-2-bromobutane.
brainly.com/question/17031230
#SPJ11