Answers
Answer:
i think your answer is d
Explanation:
sorry if it is wrong
Related Questions
where and how are chromosomes formed ? state their significance
Answers
Chromosomes are formed inside the nucleus of a cell. They carry genetic information and are responsible for traits and characteristics passed down from one generation to the next. Chromosomes play a crucial role in cell division, and chromosomal abnormalities can lead to a variety of genetic disorders.
Chromosomes are formed inside the nucleus of a cell. The number of chromosomes present in the cells is constant for a given organism, which is known as the chromosome number. The chromosomes in eukaryotic cells are formed by the condensation of chromatin fibers during cell division, and they carry genetic information in the form of genes. Chromosomes are significant for various reasons. The DNA present in chromosomes carries the genetic information necessary for the development, growth, and functioning of an organism. Chromosomes are responsible for traits and characteristics passed down from one generation to the next. In addition, chromosomes play a crucial role in cell division, ensuring that each daughter cell receives the correct amount of genetic information. Chromosomal abnormalities can lead to a variety of genetic disorders, including Down syndrome, Turner syndrome, and Klinefelter syndrome. Therefore, the formation and structure of chromosomes are important for understanding genetics and disease.For more questions on Chromosomes
https://brainly.com/question/32612200
#SPJ8
why does sodium hypochlorite have to be added slowly to borneol
Answers
Sodium hypochlorite (NaClO) should be added slowly to borenol because the reaction between sodium hypochlorite and borenol is exothermic and releases heat. If the sodium hypochlorite is added too quickly, the reaction will become very exothermic and generate a lot of heat, which could cause the reaction mixture to boil over or even explode.
By adding the sodium hypochlorite slowly, the heat generated by the reaction is dissipated gradually, preventing an uncontrolled release of energy. This helps to ensure the reaction proceeds smoothly and safely. Additionally, by adding the sodium hypochlorite slowly, the reaction can be controlled more easily, which can lead to a more efficient and complete reaction.
Learn more about sodium hypochlorite here:
https://brainly.com/question/29708118
#SPJ4
What is the structural formula of 4-methyl pentan-2-ol
Answers
The 4-methyl pentane-2-ol (\(C_6H_{14}O\)) is an alcohol compound with a methyl group attached to the fourth carbon atom and a hydroxyl group attached to the second carbon atom in a five-carbon chain.
The structural formula of 4-methyl pentane-2-ol is \(C_6H_{14}O\). This is an alcohol compound with six carbon atoms, fourteen hydrogen atoms, and one oxygen atom. The first part of the name, 4-methyl, indicates that there is a methyl group (\(CH_3\)) attached to the fourth carbon atom in the chain. Pentan-2-ol tells us that there are five carbon atoms in the chain and that the hydroxyl group (OH) is attached to the second carbon atom. Therefore, the structural formula of 4-methyl pentane-2-ol can be written as \(CH_3CH(CH_3)CH(CH_2OH)CH_2CH_3\). This can be further simplified as \(CH_3CH(CH_3)CH(CH_2OH)CH_2CH_3\)which represents the complete structural formula of 4-methyl pentan-2-ol.4-methyl pentane-2-oil is an organic compound with a wide range of applications, including as a solvent, in the manufacture of cosmetics and perfumes, and as a flavoring agent in food and beverages. Its unique structure and properties make it a valuable component in various chemical and industrial processes.For more questions on methyl group
https://brainly.com/question/31238796
#SPJ8
what is the spectator ion in this reaction: lino3(aq) na (aq) -> nano3(s) li (aq)
Answers
In the given reaction, the spectator ion is NO₃⁻ (nitrate ion).
A spectator ion is an ion that does not participate in the overall chemical reaction and remains unchanged throughout the reaction. It is present on both sides of the equation.
In this case, LiNO₃ and NaNO₃ are both soluble compounds that dissociate into their respective ions in aqueous solution. The Li⁺ (lithium ion) and Na⁺ (sodium ion) are involved in the reaction, but they are not spectator ions.
On the other hand, NO₃⁻ appears as a common ion in both the reactant (LiNO₃) and the product (NaNO₃). It does not undergo any chemical changes and remains in solution as a spectator ion.
Therefore, the spectator ion in this reaction is NO₃⁻.
To know more about spectator ion, refer here:
https://brainly.com/question/28334213#
#SPJ11
Tyrone is walking along a balance beam. He is using his
Answers
Answer:
Cerebellum
Explanation:
helps with balance, movement, and coordination.
Answer:
Cerebellum
Explanation:
A solution is made containing 8.4 g
of potassium nitrate per 125 g of
water.
What is the weight/weight % or
percent by mass of the solute?
Answers
The percent by mass : 6.3%
Further explanationThe concentration of a substance can be expressed in several quantities such as moles, percent (%) weight/volume,), molarity, molality, parts per million (ppm) or mole fraction. The concentration shows the amount of solute in a unit of the amount of solvent.
mass of potassium nitrate = 8.4 g⇒solute
mass of solution = mass of solute+mass of solvent(water)mass of potassium nitrate + mass of water = 8.4 g + 125 g =133.4 g
Percent by mass of the solute :
\(\tt =\dfrac{mass~solute}{mass~solution}\times 100\%\\\\=\dfrac{8.4}{133.4}\times 100\%\\\\=6.3\%\)
chemicals that accumulate in soil and water as a result of car emissions, improperly disposed waste, industry, and agriculture are known as
Answers
The chemicals that accumulate in soil and water as a result of car emissions, improperly disposed waste, industry, and agriculture are known as persistent organic pollutant.
What is persistent organic pollutant ?Organic molecules that are resistant to environmental deterioration through chemical, biological, and photolytic processes are persistent organic pollutants, sometimes referred to as "forever chemicals."
They are dangerous compounds that have a negative impact on the environment and human health all over the world.
POPs are compounds that are persistent in the environment, bioaccumulate in the food chain, and have the potential to have negative impacts on both human health and the ecosystem.
Thus, persistent organic pollutants are hazardous compounds that have a negative impact on the environment and human health all over the world.
To learn more about persistent organic pollutant, follow the link;
https://brainly.com/question/10269742
#SPJ1
Sugar turns into black mass on heating Give reason
need fast
Answers
The heat causes the sugar's atoms to combine with the oxygen in the air, forming new groups of atoms. Energy is released in this chemical reaction in the form of smoke and black soot. hope this helps
Sugar is made of carbon, hydrogen, and oxygen atoms. When heated over a candle, these elements react with the fire to turn into a liquid. The heat causes the sugar's atoms to combine with the oxygen in the air, forming new groups of atoms. Energy is released in this chemical reaction in the form of smoke and black soot.
If calcium chloride is dissolved in water, what can be said about the concentration of the calcium ion?.
Answers
Calcium chloride is an ionic compound made up of calcium and chloride ions. When it is dissolved in water, it dissociates into its ions. This means that it breaks apart into separate ions and becomes a solution that contains calcium and chloride ions.
The concentration of calcium ion is high in a calcium chloride solution. The concentration of calcium ions in the solution is determined by the amount of calcium chloride dissolved in the water. Calcium ions have a charge of +2, and when calcium chloride dissolves in water, it dissociates into one calcium ion and two chloride ions.
Calcium ion concentration can be measured using a variety of methods. For example, a simple method involves adding a calcium ion indicator to the solution. The indicator changes color based on the concentration of calcium ions in the solution, allowing for a visual determination of the concentration.
To know more about ionic visit:
https://brainly.com/question/29523788
#SPJ11
The gas formed when coal is heated in the absence of air_____________
Answers
describe the structure of an ionic compound
Answers
Answer:
An ionic compound is a giant structure of ions.
Explanation:
As a result of ion attraction, ions form a regular pattern with oppositely charged ions next to each other in an ionic compound. Ions are organized in an ionic network, a giant structure made up of alternating ions.
Which body system is responsible for coordinating actions such as breathing, talking, walking, and blinking?
Group of answer choices
Digestive System
Endocrine System
Nervous System
Skeletal System
Answers
nervous system helps in controlling and coordinating various activities of the human body. The three types of nerves, cranial nerves, spinal nerves and visceral nerves run through the body and help in sending and receiving messages in the form of electrical impulses.
What is the acetic acid constanta (ka)?
Answers
The value of Ka constant for acetic acid is 1.75 × 10⁻⁵.
Generally, the acid dissociation constant (Ka) is used to distinguish strong acids from weak acids. Strong acids generally have exceptionally high Ka values. The Ka value is obtained by looking at the equilibrium constant for the dissociation of the acid. The higher is the Ka, the more the acid dissociates into its ions.
Ka is defined as the acid dissociation constant whereas pKa is simply the -log of the constant Ka. Similarly, Kb is defined as the base dissociation constant, whereas pKb is the -log of the constant Kb.
Learn more about dissociation constant from the link given below.
https://brainly.com/question/28197409
#SPJ4
Ordinary hydrogen contains 99.30% of H atoms and 0.70% H atoms. Calculate the relative atomic mass of hydrogen.
Answers
The relative atomic mass of hydrogen 1.00
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit
Here given data is
99.30% of H atoms
0.70% H atoms
So we have to calculate the relative atomic mass of hydrogen. = ?
So the relative atomic mass = (isotope abundance × isotope mass) + (isotope abundance × isotope mass)
Relative atomic mass = (99.30% × 1) + (0.70% × 1)
Relative atomic mass = 1.00
Know more about hydrogen
https://brainly.com/question/25640729
#SPJ9
How do you decide between SN1 and SN2?
Answers
The two types of nucleophilic substitution reactions are SN1 and SN2. SN2 has two molecules, whereas SN1 only has one.
What is Nucleophilic substitution reaction?A nucleophilic molecule replaces a different atom or group of atoms on a molecule, known as the leaving group, in a nucleophilic substitution reaction. The substrate molecule is attacked by the nucleophilic molecule's abundant electrons.
A process in which one functional group or atom is swapped out for another negatively charged functional group or atom is known as a nucleophilic substitution reaction.
The SN1 reaction is monomolecular, whereas the SN2 reaction is bimolecular.
Any substitution reaction in which an atom or functional group is changed for one that has a single pair of electrons, a negatively charged ion, or both. The negatively charged ion or the atoms/molecules with lone pairs of electrons will be pulled to the positively charged area of an atom or complex in an effort to replace the functional group or atom already attached to the positive location.
Therefore, The two types of nucleophilic substitution reactions are SN1 and SN2. SN2 has two molecules, whereas SN1 only has one.
To know more about Nucleophilic substitution, refer to the link:
https://brainly.com/question/32657850
#SPJ6
What+is+the+empirical+formula+of+a+compound+of+uranium+and+fluorine+that+is+composed+of+67.6%+uranium+and+32.4%+fluorine+by+mass?
Answers
The empirical formula of a compound of uranium and fluorine that is composed of 67.6% uranium and 32.4% fluorine by mass is UF4.
Mass of Uranium in the compound = 67.6 g
Mass of Fluorine in the compound = 32.4 g
Step 1: Find the mass of each element.
Mass of Uranium in the compound = 67.6 g
Mass of Fluorine in the compound = 32.4 g
Step 2: Divide the mass of each element by the element's atomic mass to get the number of moles.
Atomic mass of Uranium = 238.03
Atomic mass of Fluorine = 18.9984
Moles of Uranium = 67.6 / 238.03
= 0.2840
Moles of Fluorine = 32.4 / 18.9984
= 1.7029
Step 3: Divide each element's moles by the smallest number of moles calculated.
0.2840 / 0.2840 = 11.7029 / 0.2840
= 6.01
Step 4: Round to the nearest whole number if necessary.
The ratio of Uranium to Fluorine in the compound is 1:6.01. Rounding off gives the ratio of Uranium to Fluorine as 1:6.
Step 5: Write the empirical formula.
The empirical formula of a compound of uranium and fluorine is UF4.
Learn more about empirical formula -
brainly.com/question/1603500
#SPJ11
A 5.00 gram sample of an unknown metal was placed in a beaker of boiling water (99.58oC). After five minutes it was immediately transfered from the boiling water to a calorimeter containing 50.0mL of water at 11.25oC. The final temperature of the metal-water mixture was 44.10oC. What is the specific heat of the metal?
Answers
The specific heat of the unknown metal is approximately 0.896 J/g°C.
How to calculate the specific heatTo determine the specific heat of the unknown metal, we'll use the heat exchange equation:
q = mcΔT
where q is the heat exchange, m is the mass, c is the specific heat, and ΔT is the temperature change.
Since heat gained by the metal is equal to heat lost by water, we have:
q_metal = q_water
m_metal × c_metal × ΔT_metal = m_water × c_water × ΔT_water
Given:
m_metal = 5.00 g
m_water = 50.0 mL (assuming 1 g/mL, mass is 50.0 g)
ΔT_metal = 44.10°C - 99.58°C = -55.48°C
ΔT_water = 44.10°C - 11.25°C = 32.85°C
c_water = 4.184 J/g°C (specific heat of water)
Now we can solve for c_metal:
5.00 × c_metal × (-55.48) = 50.0 × 4.184 × 32.85
c_metal = (50.0 × 4.184 × 32.85) / (5.00 × (-55.48))
c_metal ≈ 0.896 J/g°C
Learn more about specific heat at
https://brainly.com/question/21041726
#SPJ11
PLEASE ANSWER THIS FAST
Upper H Subscript 2 Baseline Upper S Upper O Subscript 4 Baseline (a q) double-headed arrow 2 Upper H Superscript + Baseline (a q) + Upper S Upper O Superscript 2 negative sign Subscript 4 Baseline (a q)
Which contain a common ion that will shift the equilibrium system represented by the equation shown? Select all that apply.
MgSO4
Na2S
HNO3
CaCl2
Answers
MgSO₄ and HNO₃ contain a common ion that will shift the equilibrium system. Therefore, option A, C is correct.
What is equilibrium?The equilibrium of a system can be defined as the state in which both the reactants and products in the chemical reactions are in concentrations that have not changed with time. The rates of reaction of the forward reactions and backward reactions are non-zero, but equal while in an equilibrium system.
By definition, a common ion can be described as an ion that enters the solution from two different sources. Solutions to which NaCl and AgCl are added also contain a common ion will be chloride Cl⁻ ion. The effect of common ions on solubility product equilibrium of the system.
The dissociation of sulphuric acid can be shown as:
H₂SO₄ → 2 H⁺ (aq) + SO₄²⁻ (aq)
MgSO₄ and H₂SO₄ have a common ion SO₄²⁻. H₂SO₄ and HNO₃ have a common ion H⁺ ion.
Learn more about equilibrium, here:
brainly.com/question/15118952
#SPJ2
Answer:
1. A, C
2. B- Equilibrium will sift to the left.
Explanation:
Where within the cell does the process of respiration take place?
Answers
Answer:
cytoplasm
Explanation:
Answer:
mitochondria
Explanation:
While most aerobic respiration (with oxygen) takes place in the cell's mitochondria, and anaerobic respiration (without oxygen) takes place within the cell's cytoplasm
Mark the statements which are correct. (Select all that apply. )
1 g = 10^3 mg
10^-3 g = 10^12 ng
1 s = 10^6 μs
1 km = 10^5 mm
1 s = 10^3 ms
Answers
All statements given in the question are incorrect except for 1 statement. The correct statement is:1 s = 10^3 ms.
In the question, we have been provided with 5 statements. We are asked to select all the correct statements from those 5 statements. Given below are for each statement:1 g = 10^3 mg:This is incorrect. 1 g is equal to 1000 mg.10^-3 g = 10^12 ng:This is incorrect. 10^-3 g is equal to 1 mg.1 km = 10^5 mm:This is incorrect. 1 km is equal to 1,000,000 mm.1 s = 10^6 μs:This is incorrect. 1 s is equal to 1,000,000 μs.1 s = 10^3 ms:This is correct. 1 s is equal to 1000 ms.Therefore, the main answer to this question is that only 1 statement is correct, which is:1 s = 10^3 ms.
Metric units are based on the power of ten. The base units of the International System of Units (SI) are the meter, kilogram, second, kelvin, ampere, mole, and candela. All other metric units can be derived from these basic units.The first unit in each conversion is in grams, seconds, or kilometers. The metric units for millimeters, microseconds, and nanograms are derived from these basic units. One gram is equal to 1000 milligrams (mg), 1 second is equal to 1000 milliseconds (ms), and 1 kilometer is equal to 1000000 millimeters (mm). 10^-3 g is equal to 1 milligram (mg), 10^6 μs is equal to 1 second (s), and 10^12 ng is equal to 1 gram (g).
To know more about except visit:
https://brainly.com/question/14400269
#SPJ11
Please help due today

Answers
What does the following energy
diagram represent?
A. Exothermic reaction
B. Activation energy
C. Endothermic reaction
D. Specific heat capacity
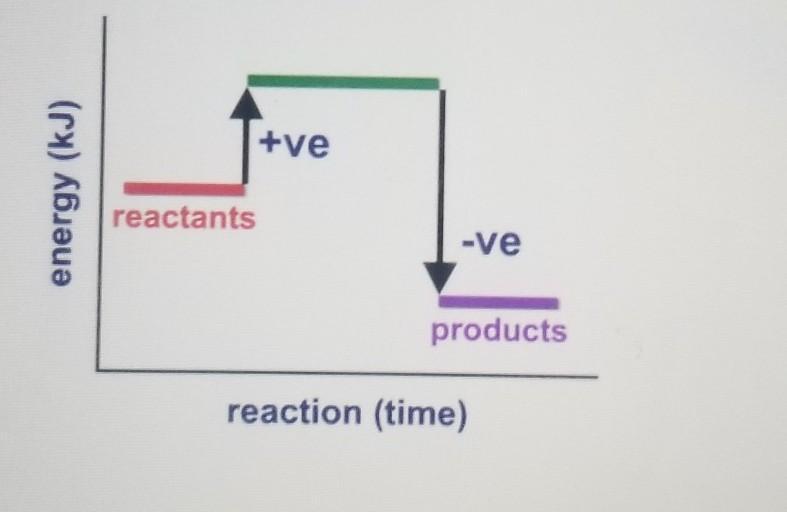
Answers
Answer:
Option A) Exothermic Reaction
Explanation:
In exothermic reaction, the energy is released. The reactants are at high energy while the products are at low energy as shown in the graph.
Answer:
A.) Exothermic reaction
Explanation:
I got it correct on founders edtell
which size of micropipette would you select to deliver 215 microliters?
Answers
The size of micropipette that you would select to deliver 215 microliters would be a micropipette with a volume range of 200-1000 microliters.
What is a micropipette?
A micropipette is a laboratory instrument used to measure and dispense small volumes of liquid, typically in the range of microliters (µL) or nanoliters (nL). They are commonly used in chemistry, biology, and biochemistry experiments, as well as in clinical and industrial settings.
Micropipettes consist of a handle, a digital or manual volume adjustment mechanism, and a tip that is placed into the liquid to be dispensed. They work by creating a vacuum or positive pressure inside the tip, which draws or pushes the liquid out of the tip.
Micropipettes come in different volume ranges and it is important to select the right one to ensure accurate delivery of the desired volume. For example, a micropipette with a volume range of 2-10 microliters would not be suitable for delivering 215 microliters, while a micropipette with a volume range of 200-1000 microliters would be more appropriate.
It's also important to note that, even if the micropipette is able to deliver 215 microliters, you should always check the calibration of the micropipette before use, to make sure that it's delivering the correct volume.
Hence, a micropipette with a volume range of 200-1000 microliters is suitable to deliver 215 microliters.
To learn more about a micropipette from the given link:
brainly.com/question/28425080
#SPJ4
Gaseous chlorine is held in two separate containers at identical temperature and pressure. The volume of container 1 is 9.4 L, and it contains 7.2 mol of the gas. The volume of container 2 is 50.1 L. How many moles of the gas are in container 2?
Answers
Answer:
Explanation:
According to Avogadro's law, equal volumes of gases at the same temperature and pressure contain the same number of molecules. Therefore, we can set up the following proportion to find the number of moles of chlorine in container 2:
Number of moles in container 1 / volume of container 1 = number of moles in container 2 / volume of container 2
We can plug in the given values:
Number of moles in container 1 = 7.2 mol
Volume of container 1 = 9.4 L
Volume of container 2 = 50.1 L
Number of moles in container 2 = x (unknown)
So we have:
7.2 mol / 9.4 L = x / 50.1 L
To solve for x, we can cross-multiply and simplify:
7.2 mol * 50.1 L = 9.4 L * x
x = (7.2 mol * 50.1 L) / 9.4 L
x = 38.2925 mol
Therefore, there are approximately 38.3 moles of chlorine in container 2.
Cloud formation takes place during which phase of the water cycle?.
Answers
Answer:
condensation stage is when clouds form
the mass of solution is equivalent to the mass of the?
A. solute + solvent
B.solvent
C.solute-solvent
D.solute
Answers
The mass of solution is equivalent to the mass of the solute and solvent.
What is solute?The material whose dissolves is known as a solute, as well as the substance
What is solvent?The solute is dissolved to produce a solution, is known as a solvent.
Solution is made by solvent , solute also. So, by adding mass of solvent and solute mass of a particular solution could be found.
It can be expressed as:
Mass of solution = mass of solute + mass of solvent
Therefore, the mass of solution is equivalent to the mass of the solute and solvent.
To know more about solute and solvent.
https://brainly.com/question/14797683
#SPJ2
An book fell from a shelf 1.5 meters from the ground. If it took the book 3 seconds to hit the ground, what was the velocity of the book?
•1 m/s
•1.5 m/s
•0.5 mls
•3 m/s
Answers
The book's initial velocity before it touches to floor is 14.2 m/s.
How does physics define velocity?The velocity of an object, which is function of time, is the rate at which its location with respect to a frame for reference changes. Similar to velocity, an item's speed and line of action (such as 60 km/h toward the north) are measured.
the book's height above the ground, h = 1.5 m
the book's rate of movement, t = 3 seconds
The following formula is used to determine the book's starting velocity:
h = ut + ¹/₂gt²
where;
The book's velocity is u.
1.5 = 3u + (0.5 x 9.8 x 3²)
1.5 = 3u + 44.1
3u = 1.5 - 44.1
3u = -42.6
\(u= \frac{-42.6}{3}\)
u = -14.2 m/s
u = 14.2 m/s downwards
Therefore, the book's initial velocity before it touches to ground equals 14.2 m/s.
To know more about velocity visit:
https://brainly.com/question/30559316
#SPJ4
URGENT! Please help! Hi, I have to do a titration lab report using the Royal Society of Chemistry online titration lab. Please help me answer the following questions using the observation table I think?


Answers
Answer:
I'm sorry, but I cannot see the observations or the data table you mentioned in your question. However, I can still provide you with some general guidance on how to approach the calculations and answer the questions based on the given information.
4. To calculate the concentration of the NaOH solution, you need to know the mass of NaOH used and the volume of the solution. The formula to calculate concentration is:
Concentration (in mol/L) = (Mass of NaOH (in grams) / molar mass of NaOH) / Volume of solution (in L)
Make sure to convert the mass of NaOH to moles by dividing it by the molar mass of NaOH. The molar mass of NaOH is the sum of the atomic masses of sodium (Na), oxygen (O), and hydrogen (H).
5. The balanced equation for the neutralization reaction between NaOH and HCl is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
(aq) represents an aqueous solution, and (l) represents a liquid.
6a. To calculate the average concentration of HCl in the sample from site B, you need to know the volumes and concentrations of the NaOH and HCl solutions used in the titration. Use the formula:
Concentration of HCl (in mol/L) = (Volume of NaOH solution (in L) * Concentration of NaOH (in mol/L)) / Volume of HCl solution (in L)
Multiply the volume of NaOH solution used by its concentration to find the amount of NaOH used. Then, divide this amount by the volume of HCl solution used to find the concentration of HCl.
6b. To determine the pH of the water at site B, you need to know the concentration of HCl from the previous calculation. The pH can be calculated using the formula:
pH = -log10[H+]
Since HCl is a strong acid, it dissociates completely into H+ ions. Therefore, the concentration of H+ ions is equal to the concentration of HCl. Take the negative logarithm (base 10) of the H+ concentration to find the pH.
To check if the water is safe, compare the calculated pH value to the range provided (pH 4.5-7.5). If the pH falls within this range, the water is considered safe for plant and animal reproduction in an aquatic environment.
6c. Use a similar calculation as in 6a to determine the average concentration of HCl in the sample from site C.
6d. Use the concentration of HCl from 6c to calculate the pH using the formula in 6b. Follow the same procedure to check if the water is safe based on the pH range.
7. To find the most current pH value for the Grand River, you can search for the latest data from reliable sources such as environmental agencies, research institutions, or government websites. Compare this pH value to the pH values obtained in the experiment to assess the difference between them.
Remember, without the specific data and observations, the calculations and comparisons provided here are only general guidelines. It's important to use the actual data from your experiment to obtain accurate results and conclusions.
Please mark as Brainliest
the maximum amount of iron(iii) hydroxide that will dissolve in a 0.218 m iron(iii) acetate solution is
Answers
The maximum amount of iron (III) hydroxide that will dissolve in a 0.218 M iron (III) acetate solution cannot be determined without additional information such as the solubility product constant (Ksp) of iron (III) hydroxide.
The solubility of a compound is determined by the product of the concentrations of its ions in solution, as governed by the solubility product constant.
Without knowing the Ksp of iron (III) hydroxide, we cannot determine the maximum amount that will dissolve in a solution. The concentration of the iron (III) acetate solution alone does not provide enough information to calculate the maximum solubility of iron (III) hydroxide.
For more questions like Solubility click the link below:
https://brainly.com/question/28170449
#SPJ11
What do you think method is faster classical or instrumental?
Answers
Answer:
Instrumental Methods.
Explanation:
Compared to simple laboratory tests, instrumental methods of analysis may give improved: speed (they are quick) accuracy (they reliably identify elements and compounds) sensitivity (they can detect very small amounts of a substance in a small amount of sample)
Have a wonderful day! :-)
