Compound F has a molecular formula of CgHgO. What is the Unsaturation Number (UN) or Double-Bond Equivalent (DBE)? Number ______
Examine the IR spectrum provided for Compound F Based on the molecular formula, the UN/DBE you calculated, and the IR spectrum, check all the functional groups that are possibly present in compound F? □ carboxylic acid □ nitrile □ ketone □ alcohol □ alkyne □ benzene ring □ ring □ alkene □ amine □ aldehyde □ ester
Answers
The molecular formula of Compound F is C₉H₉O. To calculate the Unsaturation Number (UN) or Double-Bond Equivalent (DBE), use the formula:
DBE = \(\frac{ (2C + 2 + N - H - X)}{2}\),
where C is the number of carbon atoms, N is the number of nitrogen atoms, H is the number of hydrogen atoms, and X is the number of halogen atoms.
For Compound F: DBE =\(\frac{(2 * 9 + 2 - 9 - 0)}{2} = \frac{ (18 + 2 - 9)}{2} = \frac{11}{2}\) = 5.5
Since the DBE must be a whole number, there might be a typo in the molecular formula. Assuming the correct molecular formula is C₉H₈O, the DBE would be:
DBE= \(\frac{(2 * 9 + 2 - 8 - 0) }{2} = \frac{(18 + 2 - 8) }{2} = \frac{12}{2}\) = 6
Now, based on the DBE value and the IR spectrum provided (unfortunately, we cannot see the IR spectrum, you can determine the possible functional groups present in Compound F. Some possible functional groups for a DBE of 6 are carboxylic acid, nitrile, ketone, alcohol, alkyne, benzene ring, ring, alkene, amine, aldehyde, and ester. You'll need to examine the IR spectrum to determine which of these functional groups are likely present in the compound.
for more information on Unsaturation Number : https://brainly.com/question/24432548?
#SPJ11
Related Questions
solve for x 5 to the power 2 x + 4 - 25 to the power x - 1 is equals to
Answers
Answer:
[][][][][][][][][][][][][][]
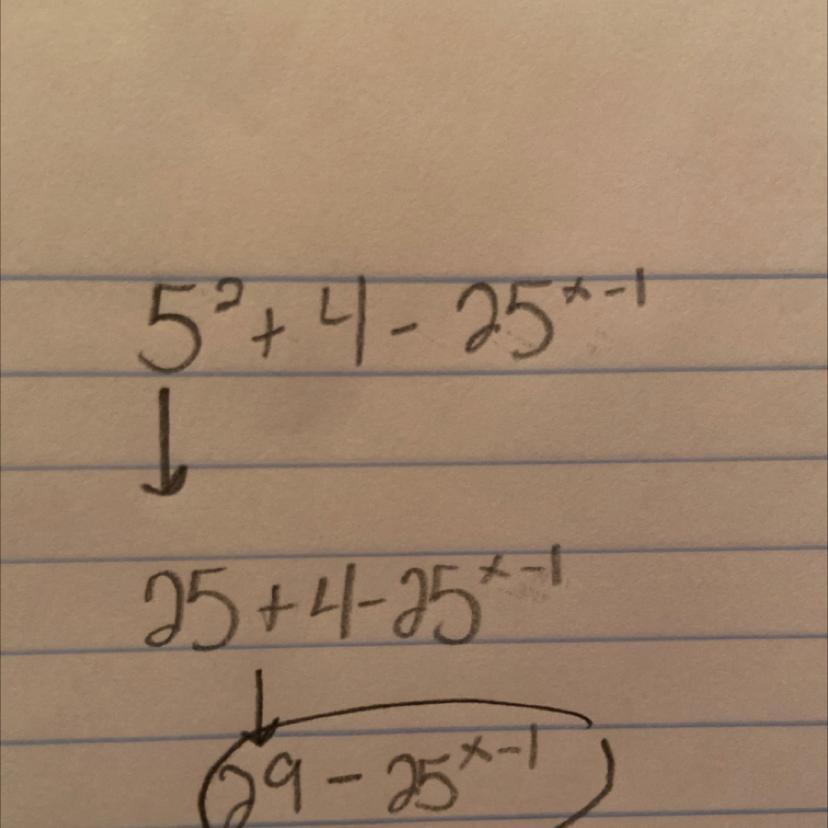
Please fine the volume please please please need asap

Answers
Answer:
64.2
Explanation:
2.8+17.8+13.7+8+21.9 = 64.2
An atom has 15 protons, 15 electrons and 16 neutrons. What is the atomic
number and mass number?
Answers
Atomic number = 15 and mass number = 31 where an atom has 15 protons, 15 electrons and 16 neutrons.
What is an atom?An atom consists of a central nucleus that is usually surrounded by one or more electrons.
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. Atomic number = Number of protons.
Mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom.
Hence, atomic number=15 and mass number=31.
Learn more about the atom here;
https://brainly.com/question/4186338
#SPJ2
Which of these materials will NOT dissolve at all in water?
A) Sugar
B) Salt crystals
C) Chalk powder
D) Detergent powder
Answers
Answer:
C) Chalk powder
Explanation:
that's why it's often used in gymnastics ~~sweaty hands yk
How can you show using Pauli's exclusion principle that p sub shell can have only 6 electrons?

Answers
where l = subshell value.
"l"values of subshell are.
s = 0.
p = 1.
d = 2.
f = 3.
So in p orbital we have 6 electrons.
what would happen to the atom calcium if you added 3 protons?
Answers
Answer:
Vanadium
Explanation:
Atomic number increases, thereby changing into an atom of Vanadium
Answer:
Vanadium
Explanation:
Science rocks
.
If the Moon orbited Earth twice as fast, how many high tides would occur in one day?
Answers
Four high tides would occur in one day, if the moon orbited Earth twice as fast.
What are high tides?The tide when the water is at its greatest elevation.
At the moment, high tides occur twice a day. So if the moon orbits the earth twice as fast, high tides will occur twice as many, which means four times a day.
High tides occur 12 hours and 25 minutes apart. It takes six hours and 12.5 minutes for the water at the shore to go from high to low, or from low to high.
The best-known effect of the moon is its gravitational pull on Earth's oceans, which results in two high tides and two low tides every day.
But if the moon were half the distance from Earth as it is now, the tides would be eight times higher.
Learn more about the high tides here:
https://brainly.com/question/24770454
#SPJ2
If the mass of the solution was 100.0g and specific heat capacity is 4.125 J/g . K and the temperature increased by 4.5 degree C due to dissolution of 0.1 mole of Na2CO3 and the calorimeter constant is 37.5 J/K. What is the molar enthalpy change
Answers
The molar enthalpy change for the dissolution of Na2CO3 is -18093.8 J/mol.
We can calculate the heat absorbed by the solution using the formula:
q = m x c x ∆T
where:
m = mass of the solution = 100.0 g
c = specific heat capacity of the solution = 4.125 J/g . K
∆T = temperature change of the solution = 4.5°C
q = 100.0 g x 4.125 J/g . K x 4.5°C
= 1846.88 J
We need to subtract the calorimeter constant from the heat absorbed by the solution to obtain the heat absorbed by the reaction:
q_rxn = q_soln - C_cal
where:
C_cal = calorimeter constant = 37.5 J/K
q_rxn = 1846.88 J - 37.5 J/K
= 1809.38 J
The molar enthalpy change (∆H) for the dissolution of Na2CO3 can be calculated using the following formula:
∆H = q_rxn / n
where:
n = moles of Na2CO3 dissolved = 0.1 mol
∆H = 1809.38 J / 0.1 mol
= -18093.8 J/mol
Note that the negative sign indicates that the reaction is exothermic (releases heat to the surroundings).
to know more about molar enthalpy refer here:
https://brainly.com/question/28590122#
#SPJ11
PLESE HELP WILL GIVE BRAINLYESTY
Imagine that you are Sir Isaac Newton. Write a short autobiographical statement that talks about who you are, where you’re from, and some of your important contributions.
Answers
Answer:
My name is Isaac Newton and I am a scientist. I am known as one of the most important scientists in history. I was born in Woolsthorpe, England on January 4, 1643. My father, a farmer who was also named Isaac Newton, had died three months before my birth. My mother remarried when I was three years old and left me in the care of my grandparents.
Explanation:
Why is reactivity with oxygen a chemical property?
Answers
The reactivity of a substance with oxygen is a chemical, not a physical property. The reason it is called a chemical property is that it relies on its electron configuration to determine how it behaves around other substances.
What is a chemical property?A chemical property is a property of any material that becomes apparent during or after a chemical reaction; that is, any quality that can only be determined by changing the chemical properties of a substance.
Oxygen is a very reactive element, is highly paramagnetic and readily combines with other elements. One of the most important chemical properties of oxygen is that it promotes combustion. Even at room temperature, oxygen binds to elements and forms e.g. rust.
Learn more about Chemical properties:
https://brainly.com/question/28308645
#SPJ1
If the centre of an atom contains 8 particle that are charged, how many particles are revolving round this centre?
Answers
Explanation:
charged particles=8 which is proton and proton=no.of electron. That's why 8 particles are revolving round this center. And this atom structure is of oxygen
If the center of an atom has 8 charged particles that are protons as the neutrons are neutral there will be 8 negative charges that are electrons revolving around the center nucleus.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether it is solid,liquid or gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged particles and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged particles and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ2
What are the physical properties of gases, and how do they change under certain conditions?
Answers
Answer: Gases expand to fill their containers, have a fluid nature, low density, can be compressed, diffuse and effuse - move in small hole spray perfume .
Explanation:
Gases have such an unpredictable nature like fluid, very low density, can be condensed, disperse and effuse move in tiny hole spray fragrance, and they dominate far more room than the fine solids out of which they establish.
Because gases are harder to identify directly, gases are defined using four physical qualities or observable characteristics such as temperature, density, particles number, and volatility.
Learn more:
https://brainly.com/question/1369730?referrer=searchResults
what element has 25 electrons transition element
Answers
Answer:
Manganese
Explanation:
Manganese is a chemical element with symbol Mn and atomic number 25. Classified as a transition metal, Manganese is a solid at room temperature. :)
When you add so much solute that no more dissolves, you have a
a.
saturated solution.
b.
unsaturated solution.
c.
neutralization.
d.
suspension.
Answers
Answer:
A
Explanation:
Calculate the approximate volume of a 0.600 mol sample of gas at 15 °C and a pressure of
1.0 atm.
Answers
1) List known values.
sample: 0.600 mol
Pressure: 1.0 atm
Temperature: 15ºC
Constant: 0.082057 L⋅atm⋅K−1⋅mol−1
List unknown values
Volume:
2) Set the equation and solve for V.
\(PV=\text{nRT}\)\(\frac{PV}{P}=\frac{nRT}{P}\)\(V=\frac{nRT}{P}\)3) Converting to proper units.
15ºC + 273.15 = 288.15 K
4) Plug in known values.
n: 0.600 mol
R: 0.082057 L⋅atm⋅K−1⋅mol−1
T: 288.15 K
P: 1.0 atm
\(V=\frac{(0.600\text{mol)}(0.082057L\cdot\text{atm}\cdot K^{-1}\cdot mol^{-1})(288.15\text{ K)}}{1.0\text{ atm}}=14.18683\text{ L}\)The sample will occupy a volume of 14.19 L.
PLS HELP FAST!!!! Determine the new concentration if you dilute 500mL of a 1.9M solution of NaCl to 1L.
Answers
Answer:
M2 = 0.95M
Explanation:
M1V1 = M2V2
(1.9)(0.5) = M2(1)
M2 = 0.95M
Consider the following potential for two inert gas (Xe) atoms at separation R : U=λe −R/rho
− R 6
A
(a) Calculate the potential energy of the two atoms at equilibrium separation R 0
. Express your answer in terms of an exponential function of (R 0
/rho). (The answer should be in the form: U= (factor) e −R 0
/rho
, and the factor should be determined. (b) If the equilibrium separation R 0
=12rho, find the equilibrium potential energy of the two atoms in terms of λ. (c) Now consider a Xe crystal with N atoms and only nearest neighbor interactions. Find the total interaction energy in units of eV/ atom assuming λ=4156eV and R 0
/rho=12
Answers
The total interaction energy in units of eV/atom assuming λ = 4156 eV and R_0/rho = 12 is 150N eV/atom.
Given Potential for two inert gas (Xe) atoms at separation R :
U=λe^(-R/rho)-R^6/a^6
a) To calculate the potential energy of the two atoms at equilibrium separation R_0,
we have to put dU/dR = 0λ e^(-R_0/rho) = (6R_0^6)/(a^6)λ e^(-R_0/rho) = (6(12rho)^6)/(a^6)
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12)
The potential energy can be expressed as, U=λe^(-R_0/rho) = ((6(12rho)^6)/(a^6)) * e^(12) * e^(-12rho/rho)= ((6*12^6)/a^6) * e^(-11rho)
b) Given R_0 = 12rho, λ = (6(12rho)^6)/(a^6) * e^(12)
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12) = (6 * 12^6)/(a^6) * e^(12) * e^(-12) = (6 * 12^6)/(a^6)
Potential energy U = λe^(-R_0/rho) = (6 * 12^6)/(a^6) * e^(-11rho)c)
The total interaction energy in units of eV/ atom assuming λ = 4156 eV and R_0/rho = 12
Therefore, λ = (6(12rho)^6)/(a^6) * e^(12) = (6 * 12^6)/(a^6) * e^(12) * e^(-12) = (6 * 12^6)/(a^6)
Total energy (U) = (N/2)U = (N/2)λe^(-R_0/rho) = (N/2)(6 * 12^6)/(a^6) * e^(-11rho) = 150N eV/atom.
Therefore, the total interaction energy in units of eV/atom assuming λ = 4156 eV and R_0/rho = 12 is 150N eV/atom.
Learn more about energy with the given link,
https://brainly.com/question/2003548
#SPJ11
calculate the concentration (m) of sodium ions in a solution made by diluting 30.0 ml of a 0.574 m solution of sodium sulfide to a total volume of 150 ml.calculate the concentration (m) of sodium ions in a solution made by diluting 30.0 ml of a 0.574 m solution of sodium sulfide to a total volume of 150 ml.1.10 m0.115 m0.230 m1.60 m0.0575 m
Answers
the concentration (m) of sodium ions in the diluted solution is approximately 0.115 M.
To calculate the concentration (m) of sodium ions in the diluted solution, we need to consider the dilution equation:
M1V1 = M2V2
Where:
M1 = initial concentration of the solution
V1 = initial volume of the solution
M2 = final concentration of the solution
V2 = final volume of the solution
Given:
M1 = 0.574 M (initial concentration)
V1 = 30.0 mL (initial volume)
V2 = 150 mL (final volume)
To find M2, we rearrange the equation:
M2 = (M1 * V1) / V2
Substituting the given values:
M2 = (0.574 M * 30.0 mL) / 150 mL = 0.1148 M
To know more about concentration visit:
brainly.com/question/30862855
#SPJ11
Acetyl-CoA from the oxidation of fatty acids can be used in the ___
Answers
Acetyl-CoA from the oxidation of fatty acids can be used in the Krebs cycle.
The Krebs cycle, also known as the citric acid cycle or tricarboxylic acid (TCA) cycle, is a series of chemical reactions that occur in the mitochondria of cells, and it plays a crucial role in cellular respiration. Acetyl-CoA, which is produced from the breakdown of glucose, amino acids, and fatty acids, enters the Krebs cycle and is further broken down to produce energy in the form of ATP.
Fatty acids are an important source of energy for the body, especially during prolonged periods of fasting or exercise. When the body needs energy, stored fats are broken down into fatty acids, which are then transported to the liver and other tissues where they are oxidized to produce acetyl-CoA. This acetyl-CoA can then enter the Krebs cycle to produce energy.
The process of fatty acid oxidation is also important for maintaining healthy blood glucose levels, as it helps to prevent the buildup of harmful fatty acids in the liver. Overall, the oxidation of fatty acids and use of acetyl-CoA in the Krebs cycle is an essential part of cellular energy metabolism.
Learn more about Krebs cycle here: https://brainly.com/question/18461491
#SPJ11
For the reaction C + 2H2 → CH4, how many grams of carbon are required to produce 7.8 moles of methane, CH4 ?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Carbon 12
Answers
Answer:
The balanced chemical equation for the reaction is:
C + 2H2 → CH4
From the equation, we can see that 1 mole of carbon reacts with 2 moles of hydrogen to produce 1 mole of methane. Therefore, to produce 7.8 moles of methane, we would need:
1 mole of carbon = 1 mole of CH4 / 2 moles of H2 = 1/2 mole of CH4
7.8 moles of CH4 = 7.8 × (1/2) moles of C = 3.9 moles of C
Now, we can use the molar mass of carbon to convert moles to grams:
Atomic mass of carbon (C) = 12.01 g/mol
3.9 moles of C × 12.01 g/mol = 46.8 g of C
Therefore, we need 46.8 grams of carbon to produce 7.8 moles of methane (CH4). Rounded to the nearest tenth, the answer is 46.8 grams.
Which sentence best explains the relationship between pressure and the
solubility of a gas?
A. The greater the pressure, the more gas that will dissolve.
B. Solubility increases with pressure for some gases but not others.
C. The lower the pressure, the more gas that will dissolve.
D. Pressure has no effect on the solubility of gases.
Answers
Answer: A. The greater the pressure, the more of gas that will dissolve.
Explanation: Increasing pressure increases the solubility of gases. It has little effect on the solubility of liquids and solids.
how many primary carbon are in 2,3 dimethylpentane
Answers
Answer:
There are 7 carbons in 2,3 dimethylpentane
Explanation:
Because 2,3-dimethlypentane is an organic compound of carbon and hydrogen with formula C7H16
If a gas is cooled from 323.0 K to 273.15 K and volume is kept constant, what final
pressure would result if the original pressure was 0.986 atm?
Answers
Explanation:
From the Ideal Gas Laws we know that
(
P
V
T
)
1
=
(
P
V
T
)
2
Inserting our given values into this equation with the correct temperature values we get:
750
m
m
H
g
⋅
V
1
323
’
K
=
P
2
⋅
V
2
273.15
’
K
;
V
1
=
V
2
P
2
=
750
⋅
(
273.15
323
)
P
2
=
634
m
m
H
g
The first step in the production of nitric acid from ammonia involves the oxidation of ammonia.
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
(a) Use standard enthalpies of formation to calculate the standard enthalpy change for this reaction.
(b) What is the change in enthalpy in the oxidation of 6.34 g of ammonia with excess oxygen?
Compound ΔHf °
NH3(g) -45.9 kJ/mol
NH3(aq) -80.3 kJ/mol
NO(g) +91.3 kJ/mol
H2O(l) -285.8 kJ/mol
H2O(g) -241.8 kJ/mol
Answers
a) Standard enthalpy change for this reaction is -902 kJ/mol.
b) The change in enthalpy in the oxidation of 6.34 g of ammonia with excess oxygen is -83.88 kJ.
Standard enthalpy change:
(a) To calculate the standard enthalpy change for the reaction, we can use the following formula:
ΔH° = ∑(ΔHf° products) - ∑(ΔHf° reactants)
Plugging in the given values, we get:
ΔH° = [4(91.3 kJ/mol) + 6(-241.8 kJ/mol)] - [4(-45.9 kJ/mol) + 5(0 kJ/mol)]
Simplifying the equation, we get:
ΔH° = -902 kJ/mol
Therefore, the standard enthalpy change for the reaction is -902 kJ/mol.
(b) To calculate the change in enthalpy for the oxidation of 6.34 g of ammonia, we need to first convert the mass of ammonia to moles. The molar mass of ammonia (NH₃) is 17.03 g/mol, so:
6.34 g NH₃ ÷ 17.03 g/mol = 0.372 mol NH₃
From the balanced equation, we can see that the reaction consumes 4 moles of NH₃ to produce 4 moles of NO. Therefore, the number of moles of NO produced by the reaction is also 0.372 mol.
Using the stoichiometry of the reaction and the standard enthalpy change calculated in part (a), we can calculate the change in enthalpy for the oxidation of 0.372 mol of NH₃:
ΔH = (ΔH° ÷ 4 mol NH₃) × 0.372 mol NH₃
Plugging in the values, we get:
ΔH = (-902 kJ/mol ÷ 4 mol NH₃) × 0.372 mol NH₃ = -83.88 kJ
Therefore, the change in enthalpy for the oxidation of 6.34 g of ammonia is -83.88 kJ.
To learn more about change in enthalpy, visit the link given below:
https://brainly.com/question/29145818
#SPJ11
20 mL of a saturated solution of KNO3 at 50 degrees Celsius is cooled to 20 degrees Celsius. Approximately what mass of solid will precipitate from this solution?
Answers
Answer:
Approximately 8.8 grams of solid KNO3 will precipitate from the solution when it's cooled from 50 degrees Celsius to 20 degrees Celsius.
Explanation:
The solubility of a solid in a liquid typically decreases as the temperature decreases. As a result, when a saturated solution is cooled, some of the solid may precipitate out of the solution.
The solubility of potassium nitrate (KNO3) in water at 20 degrees Celsius is around 42 grams per 100 milliliters of water. At 50 degrees Celsius the solubility is around 86 grams per 100 milliliters of water.
To find the mass of solid that will precipitate, we can use the difference between the solubility of KNO3 at 20 degrees Celsius and at 50 degrees Celsius.
At 20 degrees Celsius, the solubility of KNO3 is 42 g/100 mL, and at 50 degrees Celsius, the solubility is 86 g/100 mL.
So, the difference between the solubility of KNO3 at 20 degrees Celsius and at 50 degrees Celsius is 86 - 42 = 44 grams per 100 milliliters.
Since we have 20 mL of a saturated solution, we can calculate the mass of the precipitate by multiplying the difference in solubility by the volume of the solution:
44 g/100 mL * 20 mL = 8.8 grams.
So, approximately 8.8 grams of solid KNO3 will precipitate from the solution when it's cooled from 50 degrees Celsius to 20 degrees Celsius.
Please note that this is an approximate calculation and the actual amount of precipitate may vary depending on other factors such as impurities and the specific conditions of the experiment.
use the buffer equation to calculate the ph of buffer solutions prepared by dissolving the following amounts of acetic acid and sodium acetate, respectively, in enough water to make 1 l of solution: a) 0.67 moles acetic acid and 0.33 moles of sodium acetate b) 0.33 moles acetic acid and 0.67 moles of sodium acetate
Answers
A. The pH of the buffer solution is 4.38. B. pH of the buffer solution is 5.14 The buffer equation is given by: pH = pKa + log([A⁻]/[HA]), where pH is the desired pH of the buffer solution, pKa is the dissociation constant of the weak acid.
For the first solution, we have 0.67 moles of acetic acid and 0.33 moles of sodium acetate in 1 L of solution. To calculate the concentrations of the acid and the conjugate base, we first need to convert the moles to the corresponding masses:
mass of acetic acid = 0.67 mol x 60.05 g/mol = 40.23 g, mass of sodium acetate = 0.33 mol x 82.03 g/mol = 27.08 g The molarities of the acid and the conjugate base can be calculated by dividing the number of moles by the volume of the solution (1 L):
[HA] = 0.67 mol/1 L = 0.67 M, [A⁻] = 0.33 mol/1 L = 0.33 M. The dissociation constant (pKa) of acetic acid is 4.76. Substituting the values into the buffer equation, we get: pH = 4.76 + log(0.33/0.67), pH = 4.76 - 0.38 pH = 4.38. Therefore, the pH of the buffer solution is 4.38.
b) For the second solution, we have 0.33 moles of acetic acid and 0.67 moles of sodium acetate in 1 L of solution. Following the same procedure as above, we get: [HA] = 0.33 mol/1 L = 0.33 M,[A⁻] = 0.67 mol/1 L = 0.67 M
Substituting these values into the buffer equation, we get:pH = 4.76 + log(0.67/0.33) pH = 4.76 + 0.38, pH = 5.14. Therefore, the pH of the buffer solution is 5.14.
In both cases, the pH of the buffer solution is close to the pKa of acetic acid, which indicates that the buffer is working effectively to resist changes in pH when small amounts of acid or base are added.
Know more about buffer solution here:
https://brainly.com/question/24262133
#SPJ11
I neeeeed heeeeeelppppppp

Answers
Answer:
I think no. 4
Explanation:
It is true that Earth’s orbit is not a perfect circle. It is slightly elongated, so that during part of the year, Earth is closer to the Sun than at other times. However, in the Northern Hemisphere, we are having winter when Earth is closest to the Sun and summer when it is farthest away!
There is a completely different reason for Earth's seasons.
Earth's axis is an imaginary pole going right through the center of Earth from "top" to "bottom." Earth spins around this pole, making one complete turn each day. That is why we have day and night, and why every part of Earth's surface gets some of each.
Earth has seasons because its axis doesn't stand up straight
(to be clear I just copied it from some resources)
What is the formula for the compound formed when aluminum ions (Al3+) and chlorine ions (Cl-) unite?
A)AlCl
B)Al2Cl
C)AlCl2
D)AlCl3
Answers
The current day periodic table is organized by
Answers
Answer:
Dmitri Mendeleev
Explanation:
Dmitri Mendeleev published the first recognizable periodic table in 1869, developed mainly to illustrate periodic trends of the then-known elements. He also predicted some properties of unidentified elements that were expected to fill gaps within the table. Most of his forecasts proved to be correct
Answer:
Mendeleev periodic table
Explanation:
In 1869, just five years after John Newlands put forward his
Law of Octaves, a Russian chemist called Dmitri Mendeleev
published a periodic table. Mendeleev also arranged the
elements known at the time in order of relative atomic mass
c) The amount of water on Earth changes every day. true or false?
Answers
Answer:
I think it's False
Explanation:
Explanation:
The amount of water in, on, and above our planet does not increase or decrease because of the water cycle
So it's false