Consider the following chemical equilibrium: N2(g)+3H2(g) -><- 2NH(g) Now write an equation below that shows how to calculate Kp from Kc for this reaction at an absolute temperature T. You can assume T is comfortably above room temperature. If you include any common physical constants in your equation be sure you use their standard symbols, found in the ALEKS Calculator.
Answers
\(K_{p} =\frac{K_{c} }{[RT]^{2} }\)
The equilibrium constants for a perfect gaseous mixture are\(K_{p} \:and\:K_{c}\)When equilibrium concentrations are stated in atmospheric pressure, the equilibrium constant is \(K_{p}\), and when they are expressed in molarity, the equilibrium constant is \(K_{c}\)
\(N_{2}(g)\rightarrow+3H_{2} (g)\Longleftrightarrow2NH_{3}(g)\)
we need to find the relation between \(K_{p} \:and\:K_{c}\) for this balanced equation:
\(K_{p} =\frac{[P_{NH_{3} }]^2}{[P_{N_{2} }][P_{H_{2} }]^3}\:\rightarrow(1)\)
where\(P_{x}\) means partial pressure of gas \(x\).
By ideal gas equation
\(PV=NRT\\P=\frac{N}{V} RT\)
And \(\frac{N}{V}=\frac{moles}{volume} \\\) denotes concentration
\(P=[C]RT \rightarrow\) where C means the (1)
\(K_{p} =\frac{[NH_{3}] ^{2}.[RT]^{2} }{[N_{2} ] ^{2}.[RT]^{}\times[H_{2} ]^{3} [RT ]^{3} }\)
\(K_{p} =\frac{[NH_{3}] ^{2}. }{[N_{2} ] ^{}.[^{}\[H_{2} ]^{3} } \:\)\(\times\frac{1}{RT} \rightarrow(2)\)
we know for the reaction equilibrium constant in terms of concentration-
\(K_{p} =\frac{[NH_{3}] ^{2}. }{[N_{2} ] ^{}.[^{}\[H_{2} ]^{3} } \:\)
Replacing this in equation no (2),
\(K_{p} =\frac{K_{c} }{[RT]^{2} }\)
\(K_{p} ={K_{c} }{[RT]^{\triangle n} }\\\triangle n=(moles\:of \:gaseous\: products)-(moles \:of \:gaseous\:reactant)\)
Learn more about the equilibrium constants
brainly.com/question/15118952
#SPJ4
Related Questions
How is steel made from the raw product of the blast furnace known
as "pig iron"? What are the advantages of using steel?
List references used (if any were used) to answer this question.
Answers
Steel is produced from pig iron through a process known as steelmaking or iron and steel production.
The pig iron obtained from the blast furnace contains high amounts of carbon, impurities, and other elements. To convert pig iron into steel, the carbon content needs to be reduced to desired levels, and impurities must be removed.One common method of steelmaking is the basic oxygen process (BOP). In this process, pig iron is placed in a vessel called a converter, where oxygen is blown through the molten metal. The oxygen reacts with the carbon and impurities, causing them to oxidize and form gases that are released. Alloying elements and desired additives can be added at this stage to achieve specific steel properties. Another method is the electric arc furnace (EAF), where an electric arc is used to heat and melt the pig iron, allowing impurities to be oxidized and removed.The advantages of using steel are numerous. Steel is strong, durable, and versatile, making it suitable for a wide range of applications. It has high tensile strength, which means it can withstand heavy loads and pressures. Steel is also resistant to corrosion, making it ideal for construction, infrastructure, and transportation projects. It is a recyclable material, contributing to sustainability and reducing environmental impact. Additionally, steel can be fabricated into various shapes and sizes, allowing for customization and flexibility in design.References:
A. Ghosh and A. Chatterjee, Ironmaking and Steelmaking: Theory and Practice, PHI Learning, 2008.
R.H. Tupkary and V.R. Tupkary, An Introduction to Modern Iron Making, Khanna Publishers, 2010.
J.R. Davis, ed., ASM Specialty Handbook: Carbon and Alloy Steels, ASM International, 1995.
for such more questions on production
https://brainly.com/question/25597694
#SPJ8
PLEASE HELP!! ORGANIC CHEMISTRY
A sample of a diatonic gas is loaded into an evacuated bottle at STP. The 0.25 L bottle contains 1.76 grams of the unidentified gas. Calculate the molar mass of the gas. What is the identity of the diatomic gas?
Answers
Answer:
(a) 157.7 g
(b) 7.04 g/dm³
Explanation:
(a) From the question,
According to Avogadro's Law,
1 mole of every gas at STP occupies a volume of 22.4 dm³
But mass of 1 mole of the diatomic gas = molar mass of the gas.
This Implies that,
The molar mass of the gas at STP occupies a volume of 22.4 dm³
From the question,
If,
0.25 L bottle contain 1.76 g of the gas,
Therefore,
Molar mass of the gas = (1.76×22.4)/0.25
Molar mass of the gas = 157.7 g.
(b) Density of the gas = mass/volume
D = m/v
Given: m = 1.76 g, v = 0.25 L = 0.25 dm³
Therefore,
D = 1.76/0,25
D = 7.04 g/dm³
Part E
From the observations of the simulation, which strategy was most effective for quickly and efficiently producing ammonia? Why do you think this strategy is most effective?

Answers
The production of ammonia will be favored at high pressure and low temperature.
What is an exothermic reaction?The term exothermic reaction has to do with a reaction in which the forward reaction is favored at lower temperatures.
Now looking at the reaction coordinate and the equation of the reaction, we know that the production of ammonia will be favored at high pressure and low temperature.
Learn more about ammonia:https://brainly.com/question/12276882
#SPJ1
how many moles are in 22 grams of argon
Answers
Answer:
0.551 moles
Explanation:
To calculate the number of moles in 22 grams of argon, divide the mass by the molar mass:
Number of moles = Mass / Molar mass
Number of moles = 22 g / 39.95 g/mol
Number of moles ≈ 0.551 moles
Therefore, there are approximately 0.551 moles of argon in 22 grams of argon.
What type of bonding does Ir and Hg have?
Answers
Iridium forms metallic bonds, while mercury exhibits a combination of metallic and covalent bonding. These covalent interactions give rise to the low boiling point and weak intermolecular forces in liquid mercury.
Iridium (Ir) and mercury (Hg) exhibit different types of bonding based on their electronic configurations and properties.
Iridium is a transition metal belonging to Group 9 of the periodic table. It has a partially filled d-orbital in its atomic structure, which allows it to form metallic bonds. Metallic bonding occurs when the outer electrons of metal atoms are delocalized and form a "sea" of electrons that are free to move throughout the crystal lattice. This results in the characteristic properties of metals, such as high electrical and thermal conductivity, malleability, and ductility. Iridium forms metallic bonds with other iridium atoms, contributing to its solid, dense, and lustrous nature.
Mercury, on the other hand, is a unique element. It is a transition metal, but it exhibits characteristics of both metallic and covalent bonding. At room temperature, mercury exists as a liquid, which is highly unusual for a metal. This is because mercury atoms have a weak interatomic interaction, known as metallic bonding, similar to other metals. However, due to the presence of unpaired electrons in its 6s orbital, mercury can also form weak covalent bonds. These covalent interactions give rise to the low boiling point and weak intermolecular forces in liquid mercury.
In summary, iridium forms metallic bonds, while mercury exhibits a combination of metallic and covalent bonding.
For more question on bonds
https://brainly.com/question/29794367
#SPJ8
The energy related to the motion of an object is called—
Answers
3. How many grams of oxygen are required to completely react with 240g of C₂H6?
Answers
Approximately 766.08 grams of oxygen are required to completely react with 240g of C₂H₆.
To determine the amount of oxygen required to completely react with 240g of C₂H₆ (ethane), we need to set up a balanced chemical equation for the combustion of ethane.
The balanced equation for the combustion of ethane is as follows:
C₂H₆ + O₂ → CO₂ + H₂O
From the balanced equation, we can see that the stoichiometric ratio between C₂H₆ and O₂ is 1:3. This means that for every one mole of C₂H₆, three moles of O₂ are required for complete combustion.
To calculate the amount of oxygen required, we need to convert the given mass of C₂H₆ to moles using its molar mass, and then use the stoichiometric ratio to determine the moles of O₂ required. Finally, we can convert the moles of O₂ back to grams using the molar mass of oxygen.
The molar mass of C₂H₆ is calculated as follows:
(2 x atomic mass of carbon) + (6 x atomic mass of hydrogen)
(2 x 12.01 g/mol) + (6 x 1.01 g/mol) = 30.07 g/mol
Now, we can proceed with the calculation:
Calculate the moles of C₂H₆:
moles of C₂H₆ = mass of C₂H₆ / molar mass of C₂H₆
moles of C₂H₆ = 240 g / 30.07 g/mol ≈ 7.98 mol
Determine the moles of O₂ using the stoichiometric ratio:
moles of O₂ = moles of C₂H₆ x (3 moles of O₂ / 1 mole of C₂H₆)
moles of O₂ = 7.98 mol x 3 ≈ 23.94 mol
Convert moles of O₂ to grams:
mass of O₂ = moles of O₂ x molar mass of O₂
mass of O₂ = 23.94 mol x 32.00 g/mol = 766.08 g
For more such questions on oxygen visit:
https://brainly.com/question/28009615
#SPJ8
no question................................
Answers
Answer:
ok
Explanation:
The smallest possible particle of an element is a(n) .
Answers
Answer: Atoms are the smallest particles of an element.
Based on a Kc value of 0.250 and the given data table, what are the equilibrium concentrations of XY, X, and Y , respectively?
Answers
From the solution that we have in the question;
The concentration of X and Y is 0.28 MThe concentration of XY is 0.32 MWhat is the equilibrium constant?The equilibrium constant, denoted as K, is a value that quantitatively represents the ratio of the concentrations of products to reactants at equilibrium in a chemical reaction.
It is a fundamental concept in chemical equilibrium.
The value of the equilibrium constant provides valuable information about the position of equilibrium and the relative concentrations of species involved in a chemical reaction.
Kc = [X] [Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\)
\(0.25(0.5 - x) = (0.1 + x)^2\)
\(0.125 - 0.25x =0.01 + 0.2x + x^2\\ x^2 + 0.45x - 0.115 = 0\)
x = 0.18 M
The equilibrium amount of X and Y= 0.28 M and the equilibrium concentration of XY = 0.32 M
Learn more about equilibrium constant:
https://brainly.com/question/29253884
#SPJ1
Based on the answer to the question that we have;
A 0.28 M concentration of X and Y exists at equilibriumXY's concentration at equilibrium is 0.32 M.The equilibrium constantThe ratio of the product to reactant concentrations in a chemical reaction at equilibrium is represented quantitatively by the equilibrium constant, abbreviated as K.
It is a cornerstone of the theory of chemical equilibrium.
A chemical reaction's equilibrium position and the relative concentrations of the species involved can both be learned from the equilibrium constant's value.
Kc = [X][Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\\0.25(0.5 - x) = (0.1 +x)^2\\0.125 - 0.25x = 0.01 +0.2x +x^2\\= 0.18 M\)
The equilibrium concentration of;
XY =0.5 - 0.18
=0.32 M
Then the equilibrium amount of
X and Y is
0.1 + 0.18= 0.28 M.
Learn more about equilibrium:brainly.com/question/29253884
#SPJ1

PLEASE HELP!!! how do i do this, i don’t understand how to do these
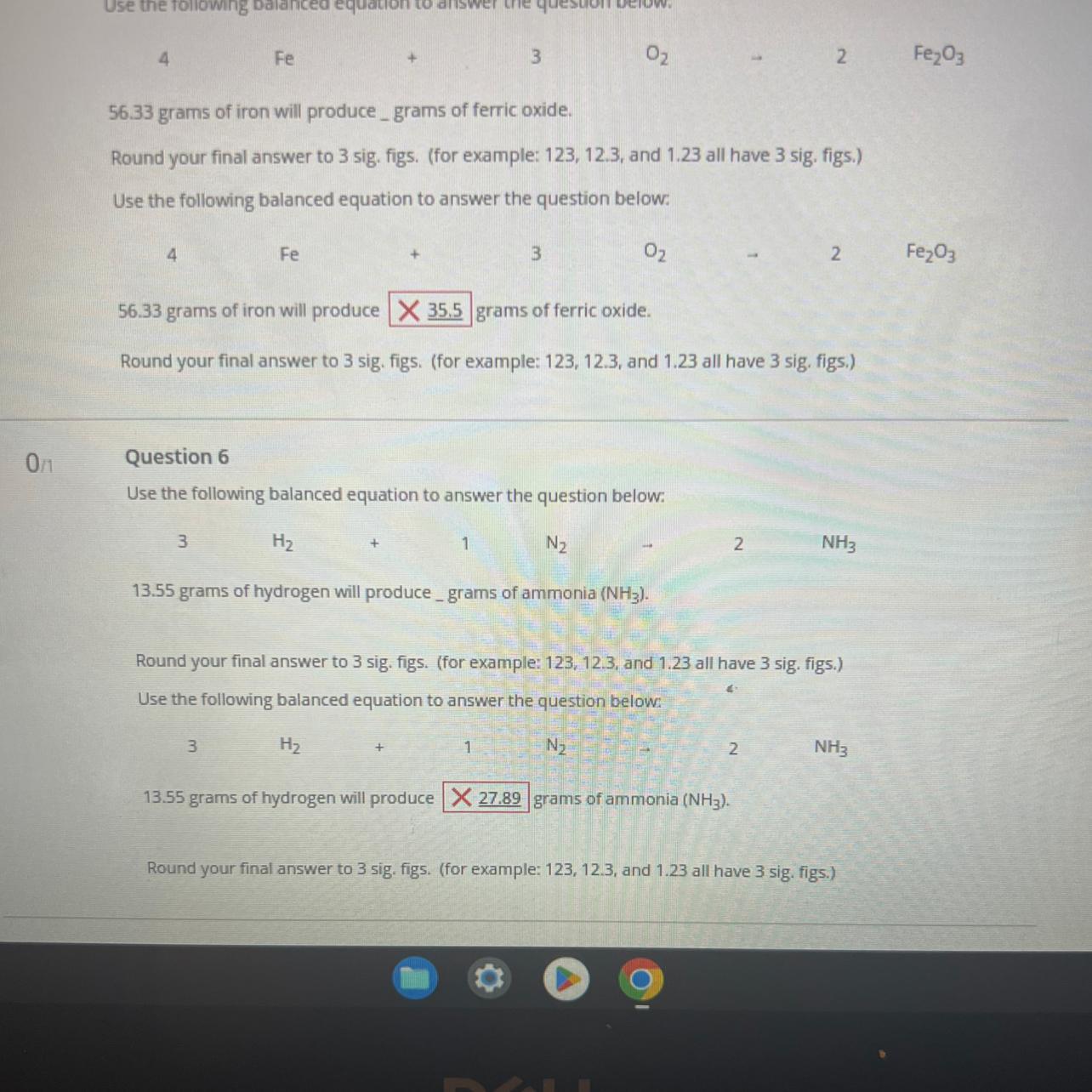
Answers
Stoichiometry is the study and calculation of quantitative (measurable) relationships of the reactants and products in chemical reactions (chemical equations).
QUESTION 5:
According to question 5, 4 moles of iron reacts with 3 moles of oxygen gas to produce 2 moles of ferric oxide.
56.33 grams of iron is equivalent to 1.01 moles
This means that 1.01 moles of iron will produce 0.51 moles of ferric oxide.
Mass of ferric oxide = 0.51 × 159.69 = 80.6 grams.
QUESTION 6:
According to question 6, 3 moles of hydrogen gas reacts with 1 mole of nitrogen gas to produce 2 moles of ammonia.
13.55 grams of hydrogen is equivalent to 6.78 moles
This means that 6.78 moles of hydrogen will produce 4.52 moles of ammonia
Mass of ammonia = 4.52 × 17 = 76.8 grams.
Learn more about mass at: https://brainly.com/question/9743981
#SPJ1
Which question is most important to developmental psychology?
A. How much do parents influence who a child becomes?
B. How can violent conflicts be prevented?
C. How does brain chemistry affect how we feel and act?
D. How can people change their thinking and behavior?
Answers
Answer:
For much of the past century, scientists studying drugs and drug use labored in the shadows of powerful myths and misconceptions about the nature of addiction. When scientists began to study addictive behavior in the 1930s, people with an addiction were thought to be morally flawed and lacking in willpower. Those views shaped society’s responses to drug use, treating it as a moral failing rather than a health problem, which led to an emphasis on punishment rather than prevention and treatment.
Today, thanks to science, our views and our responses to addiction and the broader spectrum of substance use disorders have changed dramatically. Groundbreaking discoveries about the brain have revolutionized our understanding of compulsive drug use, enabling us to respond effectively to the problem.
As a result of scientific research, we know that addiction is a medical disorder that affects the brain and changes behavior. We have identified many of the biological and environmental risk factors and are beginning to search for the genetic variations that contribute to the development and progression of the disorder. Scientists use this knowledge to develop effective prevention and treatment approaches that reduce the toll drug use takes on individuals, families, and communities.
Despite these advances, we still do not fully understand why some people develop an addiction to drugs or how drugs change the brain to foster compulsive drug use. This booklet aims to fill that knowledge gap by providing scientific information about the disorder of drug addiction, including the many harmful consequences of drug use and the basic approaches that have been developed to prevent and treat substance use disorders.
At the National Institute on Drug Abuse (NIDA), we believe that increased understanding of the basics of addiction will empower people to make informed choices in their own lives, adopt science-based policies and programs that reduce drug use and addiction in their communities, and support scientific research that improves the Nation’s well-being.
Answer:
D
Explanation:
A 1.5 kg object with a heat capacity of 6.2 J/(g oC) increases in temperature from 10.3 C to 41.9 C. How much heat was absorbed in joules?
Answers
The heat absorbed in joules is 2938800J.
The phrase "specific heat" can also refer to the ratio between a substance's specific heat capacities at a given temperature and those of a reference substance at a reference temperature, such as water at 15 °C.
In-depth measurements of heat capacity with different denominators are connected to specific heat capacity as well. Instead, the molar heat capacity, whose SI unit is the joule per kelvin per mole is obtained when the amount of substance is quantified in terms of moles.
The volumetric heat capacity, whose SI unit is joule per kelvin per cubic metre is obtained if the amount is assumed to be the volume of the sample.
It is given that in the question that
Mass of the substance = 15kg = 15000g
Specific heat of the substance = 6.2 J/ g°C
Increase in temperature from 10.3 °C to 41.9 °C
Change in temperature =41.9 °C - 10.3 °C = 31.6°C
We have to find the heat absorbed
The amount of heat (q) which is absorbed (or evolved) by a substance of mass (m) in order to bring a temperature change of (ΔT) is :
\(q = mc\)Δ\(T\) = 15000g× 6.2J/ g°C x 31.6°C = 2938800J
The heat absorbed in joules is 2938800J.
To know more about the 'specific heat' related questions
visit- https://brainly.com/question/3977170
#SPJ9
team rashta or navier ???
Answers
Answer:
navier
JAJDKEKWNEHSJJXIDJ (i need to do that so i can post the answer haha)
Answer: TEAM NAVIER FOR LIFEEEE
IF U SUPPORT TRASHTA, I HAVE NO WORDS TO DESCIRBE YOU
a 21.0 kg rock rests on the edge of a cliff 32.0 m high. how much gravitational potential energy does the rock have
Answers
Explanation:
Formula for Potential energy=mgh mass* gravity*height
21kg × 10 ×32=6720joules
If a 21.0 kg rock rests on the edge of a cliff 32.0 m high, the gravitational potential energy gained by the rock would be 6585.6 Joules.
What is mechanical energy?Mechanical energy is the combination of all the energy in motion represented by total kinetic energy and the total stored energy in the system which is represented by total potential energy.
As given in the problem a 21.0 kg rock rests on the edge of a cliff 32.0 m high, then we have to calculate the potential energy gained by the rock,
The potential energy of the rock = 21 × 9.8 × 32
= 6585.6 Joules.
Thus ,If a 21.0 kg rock rests on the edge of a cliff 32.0 m high, the gravitational potential energy gained by the rock would be 6585.6 Joules.
To learn more about mechanical energy here, refer to the link ;
brainly.com/question/12319302
#SPJ2
Researchers investigated the influence of environmental pH on the activity of peroxidase, an enzyme that catalyzes the conversion of hydrogen peroxide to water and oxygen gas. In an experiment, the researchers added a hydrogen peroxide solution containing guaiacol to several identical test tubes and adjusted the solution in each test tube to a different pH. The researchers included the guaiacol because it caused the solutions to change color as the reactions proceeded, which the researchers relied on for measuring reaction rates. Finally, the researchers added the same amount of peroxidase to each test tube and measured the rate of each reaction at 23°C. The results of the experiment are represented in Figure 1. One of the researchers proposes using oxygen gas production to measure reaction rates. Which of the following statements best justifies the use of the proposed modification as a way of creating an appropriate control for the investigation?
O the experiment can be repeated without hydrogen peroxide, which will help eliminate an uncontrolled variable. O the experiment can be repeated without hydrogen peroxide, which will help eliminate an uncontrolled variable. O the experiment can be repeated without peroxidase, which will introduce a second independent variable. O the experiment can be repeated without peroxidase, which will introduce a second independent variable. O the experiment can be repeated without guaiacol, which will reveal the effect of guaiacol on the reaction rates. O the experiment can be repeated without guaiacol, which will reveal the effect of guaiacol on the reaction rates. O the experiment can be repeated without water, which will reveal whether the reaction can occur inside a living cell.
Answers
In studying the influence of environmental pH on peroxidase activity, researchers can repeat the experiment without guaiacol, which will reveal the effect of guaiacol on reaction rates.
The proposal to use the production of gaseous oxygenOne of the investigators' proposal to use oxygen gas production to measure reaction rates leads to a change in the dependent variable.
In the first experiment the dependent variable was the volume of guaiacol and in the second experiment the dependent variable will be oxygen production.
Learn more about experiments at https://brainly.com/question/30055326
#SPJ4

6) The density of ammonia gas (NHs) in a 6.0 L container at a pressure of 820 mm Hg and a g/L.
Answers
The density of ammonia gas in the 6.0 L container at a pressure of 820 mm Hg is approximately 0.805 g/L.
To determine the density of ammonia gas (NH3) in a 6.0 L container at a pressure of 820 mm Hg, we need to use the ideal gas law equation, which relates pressure, volume, number of moles, and temperature for a given gas.
The ideal gas law equation is:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we are given the pressure (820 mm Hg), volume (6.0 L), and assuming standard temperature and pressure (STP), we can use the values for R (0.0821 L·atm/(mol·K)) and convert the pressure to atm by dividing by 760 (1 atm = 760 mm Hg).
820 mm Hg / 760 mm Hg/atm = 1.08 atm
Now we can rearrange the ideal gas law equation to solve for density (d):
d = (P * M) / (RT)
Where M is the molar mass of ammonia (NH3), which is approximately 17.03 g/mol.
Substituting the values, we have:
d = (1.08 atm * 17.03 g/mol) / (0.0821 L·atm/(mol·K) * 298 K)
Simplifying the equation, we find:
d ≈ 0.805 g/L
Therefore, the density of ammonia gas in the 6.0 L container at a pressure of 820 mm Hg is approximately 0.805 g/L.
For more question on density
https://brainly.com/question/26364788
#SPJ8
A 18.5-mL sample of a glucose IV solution has a mass of 19.1 g. What is the density of the glucose solution ?
Answers
Answer: 1.0324(g/ml)
We have the formula \(m = Vd\)
Where m is the mass, V is the volume, and d is the density
Divide both sides by V, we get \(d=\frac{m}{V}=\frac{19.1}{18.5} = 1.0324 (g/ml)\)
What element do all carbonate materials contain
Answers
Answer:
All carbonates contain one carbon atom bonded to three oxygen atoms. Carbonates may include other elements. A few are calcium, iron, and copper. Carbonate minerals are often found where seas once covered the land.
The chemist used 480g
2Fe2O3 + 3C → 4Fe + 3CO2
How many g Fe were formed?
How many g carbon needed to be taken?
How many dm3 of carbon dioxide was released during this process?
How many grams released CO2?
The chemist received 252g of Fe. Calculate the percent yield of the reaction?
Answers
The theoretical yield of Fe is 335.1 g.
54.0 g of C is needed.
The volume of CO2 produced is 0.992 L.
198.05 g of CO2 was produced.
The percent yield of the reaction is 75.2%.
The balanced chemical equation for the reaction is:
2Fe2O3 + 3C → 4Fe + 3CO2
Using the equation, we can calculate the theoretical yield of Fe and the amount of C needed.
To calculate the theoretical yield of Fe:
Convert the given mass of Fe2O3 to moles:
480 g Fe2O3 x (1 mol Fe2O3/ 160 g Fe2O3) = 3.0 mol Fe2O3
Use stoichiometry to find the moles of Fe produced:
2 mol Fe2O3 → 4 mol Fe
3.0 mol Fe2O3 x (4 mol Fe / 2 mol Fe2O3) = 6.0 mol Fe
Convert moles of Fe to grams:
6.0 mol Fe x (55.85 g Fe / 1 mol Fe) = 335.1 g Fe
To calculate the amount of C needed:
Use stoichiometry to find the moles of C needed:
2 mol Fe2O3 → 3 mol C
3.0 mol Fe2O3 x (3 mol C / 2 mol Fe2O3) = 4.5 mol C
Convert moles of C to grams:
4.5 mol C x (12.01 g C / 1 mol C) = 54.0 g C
To find the volume of CO2 produced, we need to calculate the number of moles of CO2 produced using stoichiometry.
Use stoichiometry to find the moles of CO2 produced:
2 mol Fe2O3 → 3 mol CO2
3.0 mol Fe2O3 x (3 mol CO2 / 2 mol Fe2O3) = 4.5 mol CO2
Convert moles of CO2 to volume using the ideal gas law:
PV = nRT
V = nRT/P
V = (4.5 mol) (0.0821 L atm mol^-1 K^-1) (298 K) / (1 atm)
V = 0.992 L
To find the mass of CO2 produced:
Use the molar mass of CO2 to convert from moles to grams:
4.5 mol CO2 x (44.01 g CO2 / 1 mol CO2) = 198.05 g CO2
To calculate the percent yield of the reaction:
Use the given mass of Fe (252 g) and the theoretical yield of Fe (335.1 g) to calculate the percent yield:
Percent yield = (actual yield / theoretical yield) x 100%
Percent yield = (252 g / 335.1 g) x 100%
Percent yield = 75.2%
For more question on theoretical yield click on
https://brainly.com/question/25996347
#SPJ11
A sample of helium gas at 72.0 degrees C and 1.18 atm has a volume of 1.893 L. Calculate the pressure of the helium when the volume of the sample is 1.234 L at standard temperature?
Answers
Answer:
.....
Explanation:
D0wnload Phot0Math......................
10. When dissolved in water, most Group 1 metal salts can be described as
strong electrolytes.
strong acids.
weak electrolytes.
A
B
C
D
non-electrolytes.
(1)
Answers
When dissolved in water, most Group 1 metal salts can be described as strong electrolytes.
When Group 1 metal salts are dissolved in water, they can be described as strong electrolytes. This is because Group 1 metals, such as lithium (Li), sodium (Na), potassium (K), and so on, readily lose their outermost valence electron to form positive ions (cations). These cations then dissociate completely in water, separating from the anions to which they were originally bonded.
The dissociation of Group 1 metal salts in water results in the formation of positively charged metal ions and negatively charged non-metal ions (anions). These ions are free to move and conduct electric current, making the solution a good conductor of electricity. The complete dissociation of Group 1 metal salts in water and the presence of freely moving ions make them strong electrolytes.
Strong electrolytes are substances that ionize completely or almost completely in solution, producing a high concentration of ions. This is in contrast to weak electrolytes, which only partially ionize and produce a lower concentration of ions.
In summary, when Group 1 metal salts are dissolved in water, they form strong electrolytes due to their ability to dissociate completely into ions, leading to a high concentration of freely moving ions in the solution, thus enabling efficient electrical conductivity.
Know more about Group 1 metal salts here:
https://brainly.com/question/13277375
#SPJ8
a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
Which of the following limits a population's growth?
Answers
Answer: Limitations to population growth are either density-dependant or density-independent. Density-dependent factors include disease, competition, and predation. Density-dependant factors can have either a positive or a negative correlation to population size.
Explanation:
List the 2 pKa's for H2SO4
Answers
what is carboxylic acid
Carboxylic acids.
Answers
Answer:
Carboxylic acid is an organic acid containing a carboxyl group. The simplest examples are methanoic (or formic) acid and ethanoic (or acetic) acid. It is used in the production of polymers, biopolymers, coatings, adhesives, and pharmaceutical drugs. They also can be used as solvents, food additives, antimicrobials, and flavorings.
Explanation:
Hope that helps.
C6H12O6 + 602 → 6CO2 + 6H₂O
The most efficient ratio is
1 C6H12O6 6 02.
Which set of reactants will be the most
efficient (least wasteful of materials) for
the reaction?
A. 1.0 mol C6H12O6 and 3.0 mol O₂
B. 1.5 mol C6H₁2O6 and 3.0 mol O₂
C. 3.0 mol C6H₁2O6 and 6.0 mol O₂
D. 0.5 mol C6H₁2O6 and 3.0 mol O₂
Answers
Answer:
D
Explanation:
The ratio of C6H12O6 (which will be referred to as "the carb") to oxygen is 1 to 6, so if we find an answer which has the same ratio, it should be chosen. A is 1:3
B is even worse with a ratio of the carb to oxygen of 1:2
C is the same as B, 1:2
D has a ratio of the carb to oxygen of 1:6, which is what we are looking for.
Please answer this!
What are the half-reactions for electrolytic cell with aluminum and gold
electrodes?
A. Al³+ (aq) + 3e → Al(s) and Au(s) → Au* (aq) + e
B. Al³+ (aq) + 3e → Al(s) and Aut(aq) + e¯ → Au(s)
► Au(s).
C. Al(s) → A1³+ (aq) + 3e and Au* (aq) + e →
D. Al(s) → Al³+ (aq) + 3e¯ and Au(s) → Au*(aq) + e

Answers
Classify the substances as atomic elements, molecular elements, molecular compounds, or ionic compounds.

Answers
Answer:
compounds ok I think I can't anderstand good
A chemical reaction between X and Y forms C according to the reaction below. The data for three trials to measure the
rate of this reaction are also given.
Trial
1
2
3
[X] (M)
0.01
0.01
0.02
X+Y→C
[Y] (M)
0.015
0.030
0.015
What is the rate law for this reaction?
OR=KX²M
OR=KX³M²
OR=KXM²
OR=KX²M²
Initial Rate (M/s)
7.83x10-5
BIBE
3.13x 104
1.57x10
Answers
Explanation: The rate law for a chemical reaction is an equation that relates the rate of the reaction to the concentrations of the reactants. To determine the rate law for a reaction, experiments are typically conducted with different initial concentrations of the reactants and the initial rate of the reaction is measured.
From the data provided, it appears that the reaction is of the form X + Y → C. And the concentration of X and Y are varied in three trials and the corresponding Initial rate is measured.
In the first trial, [X] = 0.01 M and [Y] = 0.015 M, and the initial rate of the reaction is 7.83x10-5 M/s.
In the second trial, [X] = 0.01 M and [Y] = 0.03 M, and the initial rate of the reaction is 3.13x104 M/s.
In the third trial, [X] = 0.02 M and [Y] = 0.015 M, and the initial rate of the reaction is 1.57x10 M/s.
Given the data, the rate law for this reaction is OR = KX²M. This is because when the concentration of X is doubled, the rate of the reaction is quadrupled, which is consistent with a rate law of the form OR = k[X]^2.