container has a volume of 28 L and a temperature of 45 ºC The volume is increased to 34 L. What is the new temperature of the gas
Answers
Answer:
386.14 K ( 113.14 ºC)
Explanation:
Initial Volume V1 = 28 L
Initial Temperature T1 = 45 + 273 = 318 K
Final Temperature T2 = ?
Final Volume V2 = 34 L
The relationship between these quantities is given by the charles law equation;
V1 / T1 = V2/ T2
T2 = V2 * T1 / V1
Inserting the values
T2 = 34 * 318 / 28
T2 = 386.14 K
Related Questions
Which pair of diseases could both be treated effectively with antibiotics
A. athletes foot and flu
B. cholera and salmonella
C. flu and scurvy
D. malaria and rickets
Imma mark the correct answer brainliest
Answers
Answer:
cholera and salmonella
Explanation:
i believe so
An isotope of platinum has 100 neutrons. What is the atomic number of this specific isotope of platinum?
100, 98, 195, 198
Answers
Answer:
isotopes of the same elements have the same atomic number
WHat are universal indicators and what are their uses
Answers
Compare the boiling point and vapor pressure of chloroform and glycerol
Answers
Answer:
Chloroform has a boiling point of 61.15 degrees Celsius and a vapor pressure of 9.5 kPa at 20 degrees Celsius. Glycerol, on the other hand, has a boiling point of 290 degrees Celsius and a vapor pressure of 0.0002 kPa at 20 degrees Celsius. Therefore, chloroform has a much lower boiling point and a much higher vapor pressure than glycerol. This means that chloroform is more volatile and evaporates more easily than glycerol.
Explanation:
Chloroform has a boiling point of 61.15 degrees Celsius and a vapor pressure of 9.5 kPa at 20 degrees Celsius. Glycerol, on the other hand, has a boiling point of 290 degrees Celsius and a vapor pressure of 0.0002 kPa at 20 degrees Celsius. Therefore, chloroform has a much lower boiling point and a much higher vapor pressure than glycerol. This means that chloroform is more volatile and evaporates more easily than glycerol.
A scientist observed that the loss of plants near a stream resulted in more soil being washed downstream. How will the excess soil that travels downstream reduce biodiversity ????
Answers
The excess soil that travels downstream will destroy the fish habitat thereby reducing the biodiversity.
Biodiversity can be defined as different forms of life found on earth.
The biodiversity of an ecosystem is very important because it shows the importance of different organisms, no matter how small, and the roles they play in the ecosystem.
The washing away of the soil into the stream will disrupt the aquatic ecosystem.
This can lead to increased pollution and sedimentation in the streams.
If the effects are not controlled, it would lead to depletion of fish species.
Therefore, the excess soil that travels downstream will destroy the fish habitat thereby reducing the biodiversity.
Learn more here:
https://brainly.com/question/17851096
Substances known as fuels have energy stored as:
chemical energy
mechanical energy
electrical energy
kinetic energy
Answers
Answer:
Chemical energy
Explanation:
The energy stored in the bonds of atoms and molecules, is chemical energy .
Stay safe stay healthy and blessed.
Have a great day !
Thank you
Answer: Chemical energy. :]
Explanation:
if 5 L of butane is reacted what volume of carbon dioxide is produced ILL GIVE BRAINLIEST
Answers
Answer: First, here is the balanced reaction: 2C4H10 + 13O2 ===> 8CO2 + 10H2O.
This says for every mole of butane burned 4 moles of CO2 are produced, in other words a 2:1 ratio.
Next, let's determine how many moles of butane are burned. This is obtained by
5.50 g / 58.1 g/mole = 0.0947 moles butane. As CO2 is produced in a 2:1 ratio, the # moles of CO2 produced is 2 x 0.0947 = 0.1894 moles CO2.
Now we need to figure out the volume. This depends on the temperature and pressure of the CO2 which is not given, so we will assume standard conditions: 273 K and 1 atmosphere.
We now use the ideal gas law PV = nRT, or V =nRT/P, where n is the # of moles of CO2, T the absolute temperature, R the gas constant (0.082 L-atm/mole degree), and P the pressure in atmospheres ( 1 atm).
V = 0.1894 x 0.082 x 273.0 / 1 = 4.24 Liters.
Explanation:
which of the following can serve as a good source of zn for the body? (you want a water soluble salt. ingestion of zn ions has been shown to have some effect in reducing the length of a cold).
Answers
Zinc sulfate (ZnSO4) can serve as a good source of zinc for the body. It is a water-soluble salt that can provide zinc ions for absorption in the gastrointestinal tract.
Ingesting zinc ions has been shown to have some effect in reducing the length of a cold. Therefore, zinc sulfate can be an effective option for boosting zinc levels in the body and potentially supporting immune function during a cold.
Learn more about gastrointestinal, click herehttps://brainly.com/question/29728753
#SPJ11
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
Consider the following representation of a reaction mechanism.
Step 1: A+A → C+E (slow)
Step 2: E + B → A+D (fast)
Overall reaction: A + B → C + D
What should appear in the rate law for the reaction above?
A) only A and B because they are reactants in the overall reaction
B) only C and C because they are products in the overall reaction
C) only A because it is the reactant in the rate-determining step
D) only E and B because they are the reactants in the rate-determining step
Answers
Answer:
The rate determining step is step 1
Explanation:
In the rate law for the given reaction,
only A appears because it is the reactant in the rate-determining step.So, option C is correct one.
Which step is rate determining steps?The slowest steps is rate determining step of the reaction.It is because in rate determining step the intermediate is formed.What is intermediate ?The short lived species formed in the mechanism of reaction is called intermediate.The intermediates is formed in slowest step because it is unstable species.What is rate law for the reaction?The rate law is also known as rate equationIt is the expression that provides relationship between rate and concentration of the reaction in a chemical reaction.The rate law is determined by the experimentally.If the reaction occurs in multi steps then the rate of the reaction is depends upon slowest step of the reaction.learn about rate law,
https://brainly.com/question/4222261
#SPJ2
__Is when water changes from a solid to a liquid
Answers
andnfndlzns d
When water changes from a solid to a liquid it is called melting.
Solid ice melts and forms into a liquid: water.
If this incorrect, please, don't refrain to tell me. Thank you.
the main site for water reabsorption along the nephron is the __________.
Answers
The main site for water reabsorption along the nephron is the renal tubules
Particularly the proximal tubule and the descending limb of the loop of Henle. As filtrate flows through the renal tubules, water and solutes are selectively reabsorbed into the bloodstream, with the majority of water reabsorption occurring in the proximal tubule. In this region, water is reabsorbed via osmosis, facilitated by the presence of aquaporin channels in the apical and basolateral membranes of the tubule cells. The descending limb of the loop of Henle is also important for water reabsorption, as it is permeable to water but not solutes, allowing for the creation of a concentration gradient that facilitates water reabsorption in the later parts of the nephron.
To know more about nephron, here
brainly.com/question/30975111
#SPJ4
Which postulate of Dalton’s atomic theory has not been disproved by further investigation? OPTIONS All matter is made of tiny particles called atoms. Atoms are indivisible and indestructible. All atoms of a given element have the same mass. Atoms of an element are all identical. Which statement below best describes the current model of the atom based on the Rutherford’s gold foil experiment? OPTIONS An atom is a solid sphere with positive and negative charges spread throughout. The atom has a neutral nucleus containing protons and neutrons, and electrons orbiting the nucleus. The atom has a small but very dense nucleus containing protons and neutrons. The atom has a dense positive nucleus that allows electrons to pass through it. Dalton noticed that 100 g of tin will combine with 13.5 g or 27 g of oxygen. He also noticed that the ratio of 13.5 to 27 is the same as the ratio of 1 to 2. This observation corresponds to which of the following conclusions?
Answers
Statement disproved by Dalton's atomic theory is atoms are indivisible and indestructible.In Rutherford’s gold foil experiment the atom has a small but very dense nucleus containing protons and neutrons.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ1
All living organisms are composed of
I need help on this, i'll give 30 pts for brainliest
Answers
Answer:
All living organisms are made up of one or more cells, which are considered the fundamental units of life. Even unicellular organisms are complex! Inside each cell, atoms make up molecules, which make up cell organelles and structures. In multicellular organisms, similar cells form tissues.
Explanation:
Answer:
All living organisms are composed of one or more cell
Explanation:
hope this helps, please mark brainliest
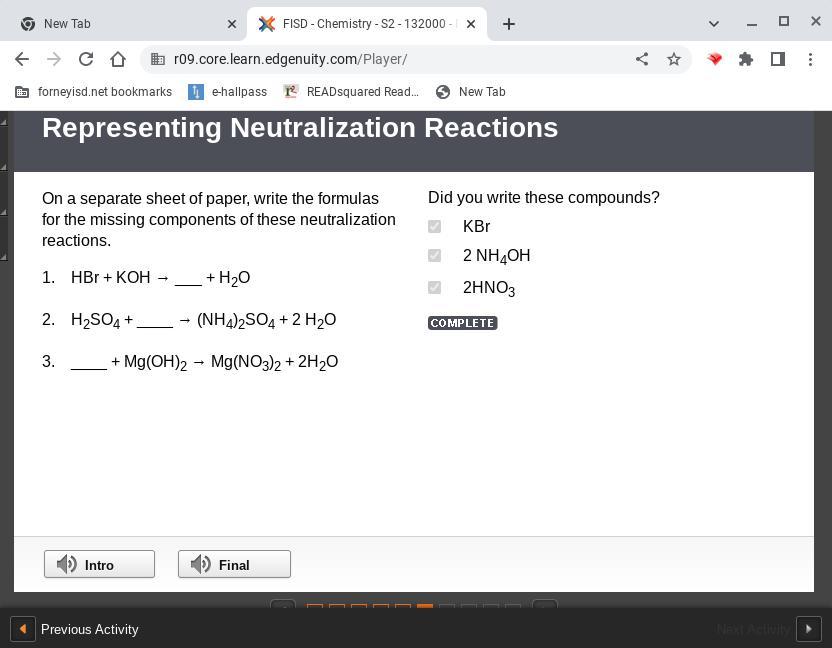
On a separate sheet of paper, write the formulas for the missing components of these neutralization reactions. 1. hbr koh → ___ h2o 2. h2so4 ____ → (nh4)2so4 2 h2o 3. ____ mg(oh)2 → mg(no3)2 2h2o
Answers
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
What is a neutralization reaction?A neutralization reaction is a reaction that occurs between an acid and a base to yield salt and water only.
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
Learn more about neutralization:https://brainly.com/question/15395418
#SPJ4
Answer:
KBr
2 NH4OH
2 HNO3
Explanation:
how is oxygen transported in the blood?multiple choice10-20% dissolved in plasma; 80-90% as oxyhemoglobin98-99% dissolved in plasma; 1-2% as oxyhemoglobin50% dissolved in plasma; 50% as oxyhemoglobin1-2% dissolved in plasma; 98-99% as oxyhemoglobin
Answers
The correct answer is A. 10-20% of oxygen is dissolved in plasma and 80-90% is transported as oxyhemoglobin in the blood.
Oxygen is transported in the blood primarily through a combination of dissolved oxygen in plasma and oxygen bound to hemoglobin within red blood cells. Approximately 10-20% of oxygen is found dissolved in plasma, while the remaining 80-90% is bound to hemoglobin in the form of oxyhemoglobin. The concentration of oxygen in plasma is determined by the partial pressure of oxygen in the environment, while the amount bound to hemoglobin is determined by the amount of hemoglobin present in the blood. Oxygen can also be transported in the form of bicarbonate and other small molecules.
learn more about bicarbonate refer: https://brainly.com/question/29855231
#SPJ11
complete question: how is oxygen transported in the blood?multiple choice
A.10-20% dissolved in plasma; 80-90% as oxyhemoglobin
B.98-99% dissolved in plasma; 1-2% as oxyhemoglobin
C.50% dissolved in plasma; 50% as oxyhemoglobin
D. 1-2% dissolved in plasma; 98-99% as oxyhemoglobin
An element has an atomic number of 76. The number of protons and electrons in a neutral atom of the element are ____.
Group of answer choices
Answers
Answer:
76 protons 76 electrons
Explanation:
The atomic number in a element is equal to the elements protons and electrons. In this case if the atomic number is 76 the number of protons and electrons would also be 76.
Hope this helps.
The resistance of a thermometer is 5 ohm at 25 degree Celsius and 6 2 at 50 degree Celsius. Using linear approximation, calculate the value of resistance temperature coefficient at 45 degree Celsius.
Answers
The approximate resistance value at 45 degrees Celsius is around 5.8 ohms.
To calculate the value of the resistance temperature coefficient at 45 degrees Celsius using linear approximation, we can use the formula:
R(T) = R0 + α(T - T0),
where R(T) is the resistance at temperature T, R0 is the resistance at a reference temperature T0, α is the resistance temperature coefficient, and (T - T0) is the temperature difference.
Given that the resistance at 25 degrees Celsius is 5 ohms (R0 = 5) and the resistance at 50 degrees Celsius is 6 ohms (R(T) = 6), we can calculate the value of α.
6 = 5 + α(50 - 25),
Simplifying the equation:
1 = 25α,
Therefore, α = 1/25 = 0.04 ohm/degree Celsius.
Using the linear approximation, we can approximate the value of the resistance at 45 degrees Celsius:
R(45) = 5 + 0.04(45 - 25) = 5 + 0.04(20) = 5 + 0.8 = 5.8 ohms.
Therefore, the value of the resistance at 45 degrees Celsius is approximately 5.8 ohms.
You can learn more about resistance at
https://brainly.com/question/17563681
#SPJ11
PLEASE HELP !!!
According to the
graph, what happens
to the concentration
of A over time?
(n) uonenu ว
Reaction: 2A A,
Time (sec)
A. It decreases and then levels out.
B. It decreases consistently.
C. It increases and then levels out.
D. It increases consistently.

Answers
According to the graph, the concentration of A decreases with time before leveling out. Option A.
Concentration of a reactant in a reversible reactionThe reaction shown is that of a reversible reaction in which A is on the reactant's side and A2 is on the product's side.
At the beginning of the reaction, the concentration of A decreases as a result of forming A2. In other words, the concentration of A2 increases just as that of A decreases.
With time, the reaction reaches an equilibrium during which the rate of formation of A equals the rate of formation of A2. At this point, the concentration of A levels off.
In summary, the concentration of A first decreases before leveling off.
More on reversible reactions can be found here: https://brainly.com/question/31950205
#SPJ1
Ethanol fuel mixtures have "E" numbers that indicate the percentage of ethanol in the mixture by volume. For example, E10 is a mixture of 10% ethanol and 90% gasoline. How much E7 should be mixed with 3000 gal of E10 to make an E9 mixture? Part: 0 / 4 Part 1 of 4 Let x represent the amount of a mixture (in gal) containing 319. ethanol. 3000 gal is the amount of E10 mixture containing 10% ethanol. Therefore, is the amount of the resulting E9 mixture containing 906 ethanol
Answers
To make an E9 mixture 8657.14 gal of E7 should be mixed with 3000 gal of E10
Given to us is the amount of ethanol in the E10 mixture is 10% of 3000 gallons:
Ethanol in E10 = 10% × 3000 gal = 0.10 × 3000 gal = 300 gal
To solve this problem, we can set up an equation based on the amount of ethanol in each mixture.
Let x represent the amount of E7 mixture (in gallons) that needs to be added to the E10 mixture to obtain the desired E9 mixture.
The amount of ethanol in the E7 mixture is 7% of x gallons:
Ethanol in E7 = 7% × gal = 0.07 × gal
The resulting E9 mixture will contain 9% ethanol of the total volume of 3000 + x gallons:
Ethanol in E9 = 9% × (3000 + x) gal = 0.09 × (3000 + x) gal
According to the problem, the resulting E9 mixture contains 906 gallons of ethanol:
Ethanol in E9 = 906 gal
Now we can set up the equation:
Ethanol in E10 + Ethanol in E7 = Ethanol in E9
300 gal + 0.07x gal = 906 gal
Subtracting 300 gal from both sides:
0.07x gal = 606 gal
Dividing both sides by 0.07:
x = 606 gal / 0.07
x = 8657.14
Therefore, approximately 8657.14 gallons of E7 mixture should be mixed with 3000 gallons of E10 to make an E9 mixture.
Learn more about ethanol mixtures here:
https://brainly.com/question/28954299
#SPJ4
Complete question: Ethanol fuel mixtures have "E" numbers that indicate the percentage of ethanol in the mixture by volume. For example, E10 is a mixture of 10% ethanol and 90% gasoline. How much E7 should be mixed with 3000 gal of E10 to make an E9 mixture?
what will happen to the electric force between two identical negative charges as they move closer together
Answers
Answer:
Like charges repel each other; unlike charges attract. Thus, two negative charges repel one another, while a positive charge attracts a negative charge.
How many grams are in 4.00 moles of Cr2O3?
Answers
Answer:
607.9616 grams
Explanation:
Hope this helped!!
how many atoms are in CuAI6PO4(OH)8
Answers
Answer: 5 atoms
Explanation:
The expression of K eq for the following reaction will not include ________. A(g) B(g) C(l) D(g)
Answers
The expression of K(eq) for the given reaction will not include C(l).
Hence, the correct option is C.
The expression of K(eq) for the given reaction will not include the concentration of the liquid phase C(l).
In the expression of the equilibrium constant, K(eq), only the concentrations of the reactant and product gases are included. The concentrations of pure solids or pure liquids are not included because they are considered to have a constant concentration and do not affect the equilibrium expression.
Therefore, in the given reaction, the expression of K(eq) will include the concentrations of A(g), B(g), and D(g) but not the concentration of C(l).
To know more about reaction here
https://brainly.com/question/13745683
#SPJ4
-- The given question is incomplete, the complete question is
"The expression of K(eq) for the following reaction will not include ________.
A(g) + B(g) = C(l) + D(g)
1.A(g) 2. B(g) 3. C(l) 4. D(g)" --
Write the IUPAC nomenclature for the following a. Al2 (CO3)3
Answers
Answer:
Aluminum carbonate | Al2(CO3)3 - PubChem
Explanation:
mark me brainliest!!
Help me answer these please!!

Answers
Answer:
Explanation:
adbc
How many ATOMS are in 7.32 moles of sulfur
dioxide?
Answers
Select all examples that apply as a physical changes in properties (please help)

Answers
George used the apparatus below to find out what substances are produced when methanol burns.
As the methanol burned two different gases were produced.
A) One of these gases condensed in the u-Tube to give a colourless liquid. Give the name of this liquid.
B) The other gas turns the limewater cloudy. Give the name of this gas

Answers
The term combustion has to do with a situation in which a substance is burnt in oxygen. When we burn a substance in oxygen, we say that the substance is oxidized.
Methanol is a member of the alkanol series of organic compounds. The equation for the combustion of methanol is shown to be as follows;
\(CH_{3} OH + 3O_{2} ---- > 2O_{2}+ 4H_{2}O\).
Looking at the set up as we can see it in the question, we can observe the following;
The gas that condensed in the u-Tube to give a colorless liquid is waterThe gas that turns limewater cloudy is carbon dioxide.Learn more about combustion:https://brainly.com/question/15117038
#SPJ1
Help question below-->

Answers
The heat transferred when 4.5 grams of Carbon reacts with H2O is approximately 42.38 kJ. Therefore, the correct option is 42 kJ absorbed.
Option B.
Given reaction is as follows: C(s) + H2O(g) + 113 kJ → CO(g) + H2(g)To find the amount of heat transferred when 4.5 grams of Carbon reacts with H2O, we have to first find the amount of moles of Carbon present. The molar mass of Carbon is 12 g/mol. Therefore, the amount of moles of Carbon can be calculated as follows:mass of carbon/molar mass of carbon=4.5 g/12 g/mol=0.375 molNow, to find the amount of heat transferred, we use the equation, q = n∆Hwhere q is the heat transferred, n is the amount of moles of Carbon present, and ∆H is the enthalpy change for the given reaction. ∆H is given in the equation as 113 kJ.To find the sign of ∆H, we look at the reactants and products. In the given reaction, Carbon reacts with H2O to form CO and H2. Since Carbon and H2O are reactants and CO and H2 are products, this reaction is an endothermic reaction. Hence, the value of ∆H is positive.∆H = 113 kJ/molNow, substituting the values in the equation, q = n∆Hq = 0.375 mol × 113 kJ/molq = 42.38 kJ (approx)
Option B.
For more questions on heat
https://brainly.com/question/30738335
#SPJ8